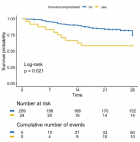
Abstract
Mini Review
Could metabolic risk factors contribute to the development of cervical cancer?
Maydelín Frontela-Noda*, Eduardo Cabrera-Rode, Maite Hernández-Menéndez and Raquel Duran-Bornot
Published: 18 December, 2019 | Volume 3 - Issue 1 | Pages: 001-006
The role of human papillomavirus infection as etiological factor for cervical squamous intraepithelial lesions and cervical cancer is well established. However, the presence of this virus is not sufficient condition for developing of cervical cancer. Currently, the contribution of other viral, environmental and host cofactors in triggering of this neoplasm is being investigated. Some metabolic risk factors have been associated with the development of several gynecological cancers such as endometrium, ovary and cervix. However, the mechanisms through which these factors contribute to carcinogenesis are complex and not fully elucidated. Few interventions regarding host metabolic factors have been performed on women at risk of developing cervical cancer. Some specific treatments and or changes in lifestyles could be carried out to avoid or delay progression to this kind of cancer. This paper aims to enlarge and update this topic based on the article ¨Association between components of the metabolic syndrome and degree of cervical squamous intraepithelial lesions in Cuban women¨, with emphasis on possible mechanisms that explain the link between central adiposity, insulin resistance and dyslipidemia with risk of premalignant lesions and cervical cancer.
Read Full Article HTML DOI: 10.29328/journal.acem.1001011 Cite this Article Read Full Article PDF
Keywords:
Cervical squamous intraepithelial lesions (SIL); Cervical cancer (CxCa); Metabolic risk factors; Central adiposity; Insulin resistance; Dyslipidemia
References
- Onwujekwe O, Samy A. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017. JAMA Oncol. 2018; 4: 1553-1568. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31560378
- Ministerio de Salud Pública. Dirección Nacional de Registros médicos y estadísticas de salud. Anuario estadístico de salud. La Habana: MINSAP. 2019. 69-101.
- Cabezas E, Camacho T, Santana A, Borrajero I, Aguilar F, et al. Programa Diagnóstico Precoz del Cáncer de Cuello del Útero en Cuba. Cuban Ministry of Public Health. 1999.
- Human papillomavirus vaccines: WHO position paper, October 2014. Wkly Epidemiol Rec. 2014; 89: 465-492. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25346960
- Prigge ES, von Knebel Doeberitz M, Reuschenbach M. Clinical relevance and implications of HPV-induced neoplasia in different anatomical locations. Mutat Res Rev Mutat Res. 2017; 772: 51-66. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28528690
- Bruni L, Barrionuevo-Rosas L, Albero G, Serrano B, Mena M, et al. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the World. 2019.
- Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, et al. Primary cervical cancer screening with human papillomavirus: End of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015; 136: 189-197. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25579108
- Soto Y, Torres G, Kourí V, Limia CM, Goicolea A, et al. Molecular Epidemiology of Human Papillomavirus Infections in Cervical Samples From Cuban Women Older Than 30 Years. J Low Genit Tract Dis. 2014; 18: 210-217. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24270200
- Soto Brito Y, Limia León CM, Kourí Cardellá V, Goicolea Maiza A, Capó de Paz V, et al. Papilomavirus humanos y otros factores asociados al desarrollo de lesiones cervicouterinas en mujeres cubanas. Panorama Cuba y Salud. 2016; 11: 24-33.
- Luhn P, Walker J, Schiffman M, Zuna RE, Dunn ST, et al. The role of co-factors in the progression from human papillomavirus infection to cervical cancer. Gynecol Oncol. 2013; 128: 265-270. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23146688
- Zhao D, Hou Z, Liu Y, Sun Q. Morbidity of metabolic syndrome ingynecologic cancers patients. Int J Clin Exp Med. 2016; 9: 336-340.
- Frontela-Noda M, Delgado DC, Cabrera-Rode E, Hernández-Menéndez M, Duran-Bornot R, et al. Association between components of the metabolic syndrome and degree of cervical squamous intraepithelial lesions in Cuban women. Diabetes Metab Syndr. 2019; 13: 1443-1448. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31336504
- Gundu HR Rao. Global Epidemic of Obesity and Diabetes: World Diabetes Day-2018. Diabetes Obes Int J. 2018; 3: 000189.
- Ethical issues in the care of the obese woman. Committee Opinion No. 600. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2014; 123: 1388-1393. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24848919
- Griffiths C, Jimenez E, Chalas E. Causal effect of obesity on gynecologic malignancies. Curr Probl Cancer. 2018; 43: 145-150. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30497850
- Chhabra S, Gangane N. Coexistence of Endometrial Cancer, Polycystic Ovarian Syndrome and Metabolic Syndrome. EC Endocrinology and Metabolic Research. 2019; 91-97.
- StocksT, Bjørge T, Ulmer H, Manjer J, Häggström C, et al. Metabolic risk score and cancer risk: pooled analysis of seven cohorts. Int J Epidemiol. 2015; 44: 1353-1363. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25652574
- Unamuno X, Gómez-Ambrosi J, Rodríguez A, Becerril S, Frühbeck G, et al. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur J Clin Invest. 2018; 48: e12997. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29995306
- Lengyel E, Makowski L, DiGiovanni J, Kolonin MG. Cancer as a Matter of Fat: The Crosstalk between Adipose Tissue and Tumors. Trends in Cancer. 2018; 4: 374-384. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29709261
- Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J Clin Oncol. 2016; 34: 4270-4276. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27903155
- Poirier P. The many paradoxes of our modern world: Is there really an obesity paradox or is it only a matter of adiposity assessment? Ann Intern Med. 2015; 163: 880-881. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26551376
- Denis GV, Obin MS. Metabolically healthy obesity: Origins and implications. Mol Aspects Med. 2013; 34: 59-70. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23068072
- Bonet-Gorbea M, Varona-Pérez P. III Encuesta Nacional de factores deriesgo y actividades preventivas de enfermedades no transmisibles. 2015.
- Lindheim SR, Welsh S, Jiang N, Hawkins A, Kellar L, et al. Trends in Management of Overweight and Obesity in Obstetrics & Gynecology,Family Medicine and Pediatrics 2011-2015. J Obes Eat Disord. 2017; 3: 1.
- US Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd edition. Washington, DC: US Department of Health and Human Services; 2018.
- Londoño-Lemos ME. Pharmacological Advances to the Treatment of Obesity. J Child Obes. 2018; 3: 3.
- Schauer DP, Feigelson HS, Koebnick C, Caan B, Weinmann S, et al. Bariatric Surgery and the Risk of Cancer in a Large Multisite Cohort. Ann Surg. 2019; 269: 95-101. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28938270
- Schutz DD, Busetto L, Dicker D, Farpour-Lambert N, Pryke R, et al. European Practical and Patient-Centred Guidelines for Adult Obesity Management in Primary Care. Obes Facts. 2019; 12: 40-66. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30673677
- Sun W, Lu J, Wu S, Bi Y, Mu Y, et al. Association of insulin resistance with breast, ovarian, endometrial and cervical cancers in non-diabetic women. Am J Cancer Res. 2016; 6: 2334-2344. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27822422
- Gallagher EJ, LeRoith D. Epidemiology and molecular mechanisms tying obesity, diabetes and the metabolic syndrome with cancer. Diabetes Care. 2013; 36: 233-239. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23882051
- Singh PJ, Alex JM, Bast F. Insulin receptor (IR) and insulin-like growth factor receptor 1 (IGF-1R) signaling systems: novel treatment strategies for cancer. Med Oncol. 2014; 31: 1-14. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24338270
- Sandford, BL, Chandler DS. The role of the insulin receptor isoforms in the Insulin-like growth factor signaling axis in cancer. Clin Oncol. 2017; 2: 1-4.
- Pickard A, Durzynska J, McCance DJ, Barton ER. The IGF axis in HPV associated cancers. Mutat Res Rev Mutat Res. 2017; 772: 67-77. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28528691
- Lee SW, Lee SY, Lee SR, Ju W, Kim SC. Plasma levels of insulin-like growth factor-1 and insulin-like growth factor binding protein-3 in women with cervical neoplasia. J Gynecol Oncol. 2010; 21: 174-180. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20922140
- Serrano ML, Romero A, Cendales R, Sanchez-Gomez M, Bravo MM. Serum levels of insulin-like growth factor-I and -II and insulin-like growth factor binding protein 3 in women with squamous intraepithelial lesions and cervical cancer. Biomedica. 2006; 26: 258-268. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16925098
- Landt S, Wehling M, Heidecke H, Jeschke S, Korlach S, et al. Prognostic significance of angiogenic factors in uterine cervical cancer. Anticancer Res. 2011; 31: 2589-2595. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21778309
- Mathur SP, Mathur RS, Creasman WT, Underwood PB, Kohler M. Early non-invasive diagnosis of cervical cancer: beyond Pap smears and human papilloma virus (HPV) testing. Cancer Biomark. 2005; 1: 183-191. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17192039
- Mannhardt B, Weinzimer SA, Wagner M, Fiedler M, Cohen P, et al. Human papillomavirus type 16 E7 oncoprotein binds and inactivates growth-inhibitory insulin-like growth factor binding protein 3. Mol Cell Biol. 2000; 20: 6483-6495. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10938125
- Pickard AS, McDade SS, McFarland SM, McCluggage WG, Wheeler CM, et al. HPV16 Down-Regulates the Insulin-Like Growth Factor Binding Protein 2 to Promote Epithelial Invasion in Organotypic Cultures. PLOS Pathogens. 2015; 11: e1004988. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26107517
- Vidal AC, Henry NM, Murphy SK, Oneko O, Nye M, et al. PEG1/MEST and IGF2 DNA methylation in CIN and in cervical cancer. Clin Transl Oncol. 2014; 16: 266-272. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23775149
- Soto D, Song C, McLaughlin-Drubin ME. Epigenetic Alterations in Human Papilloma virus: Associated Cancers. Viruses. 2017; 9: 1-18. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28862667
- Leick MB, Shoff CJ, Wang EC, Congress JL, Gallicano GI. Loss of imprinting of IGF2 and the epigenetic progenitor model of cancer. Am J Stem Cell. 2012; 1: 59-74. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23671798
- Taheri M, Ghafouri-Fard S. Long Non-Coding RNA Signature in Cervical Cancer. Klin Onkol. 2018; 31: 403-408. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30319271
- Baylin SB; Jones PA. A decade of exploring the cancer epigenome-Biological and translational implications. Nat Rev Cancer. 2011; 11: 726-734. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21941284
- Van der Veeken J, Oliveira S, Schiffelers RM, Storm G, Van Bergen En Henegouwen PM, et al. Crosstalk between epidermal growth factor receptor- and insulin-like growth factor-1 receptor signaling: implications for cancer therapy. Curr Cancer Drug Targets. 2009; 9: 748-760. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19754359
- Tseng CH. Metformin use and cervical cancer risk in female patients with type 2 diabetes. Oncotarget. 2016; 7: 59548-59555. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27486978
- Usman H, Munir R, Ameer F, Hasnain S. Cancer associated dyslipidemia. Adv in Dyslipidemia. 2016; 2: 32.
- Melvin JC, Holmberg L, Rohrmann S, Loda M, Van Hemelrijck M. Serum lipid profiles and cancer risk in the context of obesity: four meta-analyses. J Cancer Epidemiol. 2013; 2013: 823849. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23401687
- Chandler PD, Song Y, Lin J, Zhang S, Sesso HD, et al. Lipid biomarkers and long-term risk of cancer in the Women’s Health Study. Am J Clin Nutr. 2016; 103: 1397-1407. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27099252
- Katzke VA, Sookthai D, Johnson T, Kühn T, Kaaks R. Blood lipids and lipoproteins in relation to incidence and mortality risks for CVD and cancer in the prospective EPIC–Heidelberg cohort. BMC Medicine. 2017; 15: 218. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29254484
- Ulmer H, Bjorge T, Concin H, Lukanovae A, Manjerf J, et al. Metabolic risk factors and cervical cancer in the metabolic syndrome and cancer project (Me-Can). Gynecol Oncol. 2012; 125: 330-335. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22330614
- Ahn HK, Shin JW, Ahn HY, CY Park, NW Lee, et al. Metabolic components and recurrence in early -stage cervical cancer. Tumor Biol. 2015; 36: 2201. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25398694
- Matsuda M, Shimomura I. Increased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis and cancer. Obes Res Clin Pract. 2013; 1-12. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24455761
- Lewis GF. Determinants of plasma HDL concentrations and reverse cholesterol transport. Curr Opin Cardiol. 2006, 21: 345-352. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16755204
- Lofterød T, Mortensen ES, Nalwoga H, Wilsgaard T, Frydenberg H, et al. Impact of pre-diagnostic triglycerides and HDL-cholesterol on breast cancerrecurrence and survival by breast cancer subtypes. BMC Cancer. 2018; 18: 654. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29902993
- Balaban S, Lee LS, Schreuder M, Hoy AJ. Obesity and cancer progression: Is there a role of fatty acid metabolism? BioMed Res Int. 2015; 2015: 1-17. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25866768
- Carter JC, Church FC. Mature breast adipocytes promote breast cancer cell motility. Experim and Mol Pathol. 2012; 92: 312-317. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22445926
- Agarwal AK, Garg A. Enzymatic activity of the human 1-acylglycerol-3-phosphate-????-acyltransferase isoform 11: upregulated in breast and cervical cancers. J of Lip Res. 2010; 51: 2143-2152. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20363836
- Zamanian-Daryoush M, DiDonato JA. Apolipoprotein A-I and cancer. Front Pharmacol. 2015; 6: 265. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26617517
- Soran H, Hama S, Yadav R, Durrington PN. HDL functionality. Curr OpinLipidol. 2012; 23: 353-366. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22732521
- Von Eckardstein A, Hersberger M, Rohrer L. Current understanding of themetabolism and biological actions of HDL. Curr Opin Clin Nutr Metab Care. 2005; 8:147-152. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15716792
- Annema W, von Eckardstein, A. Dysfunctional high-density lipoproteins in coronary heart disease: Implications for diagnostics and therapy. Transl Res. 2016; 173: 30-57. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26972566
- Moriyama K, Negami M, Takahashi E. HDL2-cholesterol/HDL3-cholesterol ratio was associated with insulin resistance, high-molecular-weight adiponectin, and components for metabolic syndrome in Japanese. Diabetes Res Clin Pract. 2014; 106: 360-365. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25201260
- Pérez-Méndez Ó, Pacheco HG, Martínez-Sánchez C, Franco M. HDL-cholesterol in coronary artery disease risk: Function or structure? Clin Chim Acta. 2014; 429: 111-122. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24333390
- McGrowder D, Riley C, Morrison EY, Gordon L. The role of high-density lipoproteins in reducing the risk of vascular diseases, neurogenerative disorders and cancer. Cholesterol. 2010; 2011, 496925. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21490772
- Estrada-Luna D, Ortiz-Rodriguez MA, Medina-Briseño L, Carreón-Torres E, Izquierdo-Vega JA, et al. Current Therapies Focused on High-Density Lipoproteins Associated with Cardiovascular Disease. Molecules. 2018; 23: 2730. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30360466
Similar Articles
-
Could metabolic risk factors contribute to the development of cervical cancer?Maydelín Frontela-Noda*,Eduardo Cabrera-Rode,Maite Hernández-Menéndez,Raquel Duran-Bornot. Could metabolic risk factors contribute to the development of cervical cancer?. . 2019 doi: 10.29328/journal.acem.1001011; 3: 001-006
-
Evaluation of endothelial function in obese children and adolescentsHacer Efnan Melek,Ayça Törel Ergür*,Gökçe Kaan Ataç. Evaluation of endothelial function in obese children and adolescents. . 2021 doi: 10.29328/journal.acem.1001019; 5: 014-023
-
Metabolic syndrome: A case reportDragan Klaric,Marta Martinis*,Marta Klaric. Metabolic syndrome: A case report. . 2021 doi: 10.29328/journal.acem.1001022; 5: 031-035
Recently Viewed
-
Pancreatico-gastric FistulaRony Varghese*, Amal Upadhyay, Pawan Kumar Jaiprakah Maniyar. Pancreatico-gastric Fistula. J Clin Med Exp Images. 2024: doi: 10.29328/journal.jcmei.1001030; 8: 001-002
-
Transumbilical Single-incision Hiatal Hernia Repair and Nissen Fundoplication in situs Inversus Totalis: A Rare Case ReportQing Cao,Chen Kang,Kang Gu,Yin Peng,Yang Lv,Xu-Zhong Ding,Peng Li*. Transumbilical Single-incision Hiatal Hernia Repair and Nissen Fundoplication in situs Inversus Totalis: A Rare Case Report. Adv Treat ENT Disord. 2026: doi: 10.29328/journal.ated.1001017; 10: 001-003
-
Intravenous Leiomyomatosis of the Uterus with Intracardiac ExtensionTomas Reyes-del Castillo*,Minerva I Hernandez-Rejon,Jose L Ruiz-Pier,Mario Peñaloza-Guadarrama,Carlos E Merinos-Avila,Cristina Juarez-Cabrera,Pedro A del Valle-Maldonado,Sofia Ley-Tapia,Valentín Gonzalez-Flores. Intravenous Leiomyomatosis of the Uterus with Intracardiac Extension. Arch Vas Med. 2025: doi: 10.29328/journal.avm.1001021; 9: 003-007
-
Identification, Molecular Confirmation, and Antibiotic Sensitivity of Bacteria Isolated from Repeat Breeder Cows in Rangpur DivisionMst. Mousumi Afroj,Md Faruk Islam,Muhammad Mamunur Roshid,Md Shanto Hossain,Md Samiul Tousif,Omiaya Azam Oishi,Subroto Sarma,Begum Fatema Zohara*. Identification, Molecular Confirmation, and Antibiotic Sensitivity of Bacteria Isolated from Repeat Breeder Cows in Rangpur Division. Insights Vet Sci. 2025: doi: 10.29328/journal.ivs.1001047; 9: 008-17
-
Adalimumab in the Treatment of Complex Sarcoidosis-related Inflammatory Eye Disease: A Case SeriesMina Al-Awqati, Supritha Prasad*, Valeria Esparza, Jacqueline Jansz, Wuily Carpio, Christian Ascoli, Huan Chang, Pooja Bhat, Ann-Marie Lobo-Chan, Nadera Sweiss. Adalimumab in the Treatment of Complex Sarcoidosis-related Inflammatory Eye Disease: A Case Series. Arch Vas Med. 2024: doi: 10.29328/journal.avm.1001018; 8: 001-003
Most Viewed
-
Impact of Latex Sensitization on Asthma and Rhinitis Progression: A Study at Abidjan-Cocody University Hospital - Côte d’Ivoire (Progression of Asthma and Rhinitis related to Latex Sensitization)Dasse Sery Romuald*, KL Siransy, N Koffi, RO Yeboah, EK Nguessan, HA Adou, VP Goran-Kouacou, AU Assi, JY Seri, S Moussa, D Oura, CL Memel, H Koya, E Atoukoula. Impact of Latex Sensitization on Asthma and Rhinitis Progression: A Study at Abidjan-Cocody University Hospital - Côte d’Ivoire (Progression of Asthma and Rhinitis related to Latex Sensitization). Arch Asthma Allergy Immunol. 2024 doi: 10.29328/journal.aaai.1001035; 8: 007-012
-
Causal Link between Human Blood Metabolites and Asthma: An Investigation Using Mendelian RandomizationYong-Qing Zhu, Xiao-Yan Meng, Jing-Hua Yang*. Causal Link between Human Blood Metabolites and Asthma: An Investigation Using Mendelian Randomization. Arch Asthma Allergy Immunol. 2023 doi: 10.29328/journal.aaai.1001032; 7: 012-022
-
An algorithm to safely manage oral food challenge in an office-based setting for children with multiple food allergiesNathalie Cottel,Aïcha Dieme,Véronique Orcel,Yannick Chantran,Mélisande Bourgoin-Heck,Jocelyne Just. An algorithm to safely manage oral food challenge in an office-based setting for children with multiple food allergies. Arch Asthma Allergy Immunol. 2021 doi: 10.29328/journal.aaai.1001027; 5: 030-037
-
Snow white: an allergic girl?Oreste Vittore Brenna*. Snow white: an allergic girl?. Arch Asthma Allergy Immunol. 2022 doi: 10.29328/journal.aaai.1001029; 6: 001-002
-
Cytokine intoxication as a model of cell apoptosis and predict of schizophrenia - like affective disordersElena Viktorovna Drozdova*. Cytokine intoxication as a model of cell apoptosis and predict of schizophrenia - like affective disorders. Arch Asthma Allergy Immunol. 2021 doi: 10.29328/journal.aaai.1001028; 5: 038-040

If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."