Abstract
Research Article
Exercise preserves pancreatic β-cell mass and function in obese OLETF rats
Jiawei Zhao, Zhihong Yang, Min He, Qinghua Wang and Renming Hu*
Published: 19 June, 2018 | Volume 2 - Issue 1 | Pages: 022-029
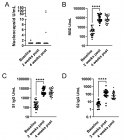
Although exercise has been proposed to be beneficial to type 2 diabetes, its effects on β-cell function and mass remain unclear. In the present study, the effects of long-term swimming training on the function and mass of β-cells in diabetic OLETF rats were examined. At 44 weeks of age after developing diabetes, the OLETF rats were divided into two groups: a control group and an exercise group. The exercise group had a daily swimming for 12 weeks. While not found with the control rats, in the obese OLETF rats, the exercise reduced the weight gain which was associated with improved glucose tolerance and elevated circulating insulin levels as determined by the oral glucose tolerance test and insulin ELISA. The exercise improved plasma total cholesterol and triglyceride levels, and also significantly increased the islet β-cell mass and pancreatic insulin content associated with decreased β-cell apoptosis and elevated activation of the serine/threonine kinase, Akt. The present studies suggest that exercise improves diabetes symptoms via enhancement of the β-cell mass and function through decreasing glucolipotoxicity and reducing β-cell apoptosis by activating Akt in obese OLETF rats.
Read Full Article HTML DOI: 10.29328/journal.acem.1001007 Cite this Article Read Full Article PDF
Keywords:
Exercise; Diabetes; Islets; β-cell mass
References
- Manson JE, Rimm EB, Stampfer MJ, Colditz GA, Willett WC, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991; 338: 774-778. Ref.: https://tinyurl.com/y8dz94fg
- Pan XR, Li GW, Hu YH, Wang JX, Yang WY, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997; 20:537- 544. Ref.: https://tinyurl.com/y9657hcr
- Pold R, Jensen LS, Jessen N, Buhl ES, Schmitz O, et al. Long-term AICAR administration and exercise prevents diabetes in ZDF rats. Diabetes. 2005; 54: 928-934. Ref.: https://tinyurl.com/yad8kvje
- Mizuno A, Noma Y, Kuwajima M, Murakami T, Zhu M, et al. Changes in islet capillary angioarchitecture coincide with impaired B-cell function but not with insulin resistance in male Otsuka-Long-Evans-Tokushima fatty rats: dimorphism of the diabetic phenotype at an advanced age. Metabolism. 1999; 48:477- 483. Ref.: https://tinyurl.com/y9ldt4of
- Tomoyuki Nishimoto, Yuichiro Amano, Ryuichi Tozawa, Eiichiro Ishikawa, Yoshimi Imura, et al. Lipid-lowering properties of TAK-475, a squalene synthase inhibitor, in vivo and in vitro. Br J Pharmacol. 2003; 139: 911- 918. Ref.: https://tinyurl.com/yd7w9u2m
- Wang Q, Brubaker PL. Glucagon-like peptide-1 treatment delays the onset of diabetes in 8 week-old db/db mice. Diabetologia. 2002; 45: 1263-1273. Ref.: https://tinyurl.com/ydyxthup
- Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S. Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology. 1997; 138: 1736-1741. Ref.: https://tinyurl.com/ybpyqusq
- Beck-Nielsen H, Pedersen O, Lindskov HO. Normalization of the insulin sensitivity and the cellular insulin binding during treatment of obese diabetics for one year. Acta Endocrinol (Copenh). 1979; 90: 103-112. Ref.: https://tinyurl.com/y74zrm7u
- Freidenberg GR, Reichart D, Olefsky JM, Henry RR. Reversibility of defective adipocyte insulin receptor kinase activity in non-insulin-dependent diabetes mellitus. Effect of weight loss. J Clin Invest. 1988; 82: 1398-1406. Ref.: https://tinyurl.com/yb8kyl4z
- Manson JE, Nathan DM, Krolewski AS, Stampfer MJ, Willett WC, et al. A prospective study of exercise and incidence of diabetes among US male physicians. JAMA. 1992; 268: 63-67. Ref.: https://tinyurl.com/y9ztz3as
- Crawford DA, Jeffery RW, French SA. Television viewing, physical inactivity and obesity. Int J Obes Relat Metab Disord. 1999; 23: 437-440. Ref.: https://tinyurl.com/yddx4l6q
- Martínez-González MA, Martínez JA, Hu FB, Gibney MJ, Kearney J, et al. Physical inactivity, sedentary lifestyle and obesity in the European Union. Int J Obes Relat Metab Disord. 1999; 23: 1192-1201. Ref.: https://tinyurl.com/ybwsdbqq
- Youngren JF, Keen S, Kulp JL, Tanner CJ, Houmard JA, et al. Enhanced muscle insulin receptor autophosphorylation with short-term aerobic exercise training. Am J Physiol Endocrinol Metab. 2001; 280: 528-533. Ref.: https://tinyurl.com/ydf5dmb4
- Holten MK, Zacho M, Gaster M, Juel C, Wojtaszewski JF, et al. Strength training increases insulin-mediated glucose uptake, GLUT4 content, and insulin signaling in skeletal muscle in patients with type 2 diabetes. Diabetes. 2004; 53: 294-305. Ref.: https://tinyurl.com/y9srdo59
- Saltin B, Helge JW. Metabolic capacity of skeletal muscles and health. Ugeskr Laeger. 2000; 162: 2159-2164. Ref.: https://tinyurl.com/y9hrawkw
- Ivy JL, Zderic TW, Fogt DL. Prevention and treatment of non-insulin-dependent diabetes mellitus. Exerc Sport Sci Rev. 1999; 27: 1-35. Ref.: https://tinyurl.com/ybnh39mu
- Donath MY, Ehses JA, Maedler K, Schumann DM, Ellingsgaard H, et al. Mechanisms of beta-cell death in type 2 diabetes. Diabetes. 2005; 2: 108-113. Ref.: https://tinyurl.com/y7jdlfgo
- Bonner-Weir S. Life and death of the pancreatic beta cells. Trends Endocrinol Metab. 2000; 11: 375-378. Ref.: https://tinyurl.com/ybfhnrtp
- Pick A, Clark J, Kubstrup C, Levisetti M, Pugh W, et al. Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the male Zucker diabetic fatty rat. Diabetes. 1998; 47: 358- 364. Ref.: https://tinyurl.com/y9w6edoh
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA , et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003; 52: 102-110. Ref.: https://tinyurl.com/y75wps4e
- El-Assaad W, Buteau J, Peyot ML, Nolan C, Roduit R, et al. Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology. 2003; 144: 4154-4163. Ref.: https://tinyurl.com/y7cyyt4t
- Dickson LM, Rhodes CJ. Pancreatic beta-cell growth and survival in the onset of type 2 diabetes: a role for protein kinase B in the Akt? Am J Physiol Endocrinol Metab. 2004; 287: 192-198. Ref.: https://tinyurl.com/y7m4gn56
- Qi J, Yang B, Ren C, Fu J, Zhang J. Swimming Exercise Alleviated Insulin Resistance by Regulating Tripartite Motif Family Protein 72 Expression and AKT Signal Pathway in Sprague-Dawley Rats Fed with High-Fat Diet. J Diabetes Res. 2016; Ref.: https://tinyurl.com/ybs47p62
- White MF. IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab. 2002; 283: 413-422. Ref.: https://tinyurl.com/y94ugynx
- Wang Q, Li L, Xu E, Wong V, Rhodes C, et al. Glucagon-like peptide-1 regulates proliferation and apoptosis via activation of protein kinase B in pancreatic INS-1 beta cells. Diabetologia. 2004; 47: 478-487. Ref.: https://tinyurl.com/ybefeuxv
- Wrede CE, Dickson LM, Lingohr MK, Briaud I, Rhodes CJ. Protein kinase B/Akt prevents fatty acid-induced apoptosis in pancreatic beta-cells (INS-1). J Biol Chem. 2002; 277: 49676-49684. Ref.: https://tinyurl.com/ydbaaach
- Elghazi L, Balcazar N, Bernal-Mizrachi E. Emerging role of protein kinase B/Akt signaling in pancreatic beta-cell mass and function. Int J Biochem Cell Biol. 2006; 38:157-163. Ref.: https://tinyurl.com/yb84boxp
- Zhao J, Zhang N, He M, Yang Z, Tong W, et al. Increased β-cell apoptosis and impaired insulin signaling pathway contributes to the onset of diabetes in OLETF rats. Cell Physiol Biochem. 2008; 21: 445-454. Ref.: https://tinyurl.com/yb9bbj9d
Figures:

Figure 1
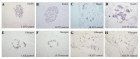
Figure 2
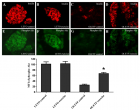
Figure 3
Similar Articles
-
Diabetes and red blood cell parametersMd. Sadikuj Jaman*,Md. Sohanur Rahman,Rubaiya Rafique Swarna,Joyanto Mahato,Md. Milon Miah,Mosa. Ayshasiddeka. Diabetes and red blood cell parameters. . 2018 doi: 10.29328/journal.acem.1001004; 2: 001-009
-
New pharmacological strategies in some metabolic endocrine disorder under a toxicological approachLuisetto M*,Ghulam Rasool Mashori,Cabianca luca. New pharmacological strategies in some metabolic endocrine disorder under a toxicological approach. . 2018 doi: 10.29328/journal.acem.1001006; 2: 015-021
-
Exercise preserves pancreatic β-cell mass and function in obese OLETF ratsJiawei Zhao,Zhihong Yang,Min He,Qinghua Wang,Renming Hu*. Exercise preserves pancreatic β-cell mass and function in obese OLETF rats. . 2018 doi: 10.29328/journal.acem.1001007; 2: 022-029
-
Comparison of Efficacy and Safety of Hydroxychloroquine and Teneligliptin in Type 2 Diabetes Patients who are Inadequately Controlled with Glimepiride, Metformin and Insulin therapy: A Randomized Controlled Trial with Parallel Group DesignPrakash Ranjan,Sajjad Ahsan*,Rabi Bhushan,Bipin Kumar,Tushar,Anup Kumar Gupta,Anand Kumar Verma,Mukesh Jain. Comparison of Efficacy and Safety of Hydroxychloroquine and Teneligliptin in Type 2 Diabetes Patients who are Inadequately Controlled with Glimepiride, Metformin and Insulin therapy: A Randomized Controlled Trial with Parallel Group Design. . 2018 doi: 10.29328/journal.acem.1001009; 2: 033-040
-
Type 2 diabetes and cancerSnobia Munir*,Samreen Riaz. Type 2 diabetes and cancer. . 2020 doi: 10.29328/journal.acem.1001012; 4: 001-006
-
Risk Factors Associated to Patients with Type 2 Diabetes in Lahore DistrictSnobia Munir*,Samreen Riaz,Tooba Arshad,Aasma Riaz. Risk Factors Associated to Patients with Type 2 Diabetes in Lahore District. . 2020 doi: 10.29328/journal.acem.1001014; 4: 011-019
-
Management of gestational diabetes during ‘COVID19 time’Nicolina Di Biase*. Management of gestational diabetes during ‘COVID19 time’. . 2020 doi: 10.29328/journal.acem.1001015; 4: 020-022
-
Evaluation of endothelial function in obese children and adolescentsHacer Efnan Melek,Ayça Törel Ergür*,Gökçe Kaan Ataç. Evaluation of endothelial function in obese children and adolescents. . 2021 doi: 10.29328/journal.acem.1001019; 5: 014-023
-
Metabolic syndrome: A case reportDragan Klaric,Marta Martinis*,Marta Klaric. Metabolic syndrome: A case report. . 2021 doi: 10.29328/journal.acem.1001022; 5: 031-035
-
Overlap of two unusual condition in childhood: hibernoma and central diabetes insipidusKübra ARSLAN*,Ayça TÖREL ERGÜR,Mehmet Ali YİNANÇ. Overlap of two unusual condition in childhood: hibernoma and central diabetes insipidus. . 2022 doi: 10.29328/journal.acem.1001023; 6: 001-003
Recently Viewed
-
Phenotypic differences in Obese Patients with Heart Failure with Preserved Ejection Fraction (HFpEF) - A Mini ReviewMichelle Nanni*, Vivian Hu, Swagata Patnaik, Alejandro Folch Sandoval, Johanna Contreras. Phenotypic differences in Obese Patients with Heart Failure with Preserved Ejection Fraction (HFpEF) - A Mini Review. New Insights Obes Gene Beyond. 2024: doi: 10.29328/journal.niogb.1001020; 8: 001-005
-
Stone on the Mesh: Intravesical Erosion after Laparoscopic Promontofixation-A Hidden Cost of DurabilityAyoub Mamad*,Mohammed Amine Bibat,Mohammed Amine Elafari,Midaoui Moncef,Amine Slaoui,Tarik Karmouni,Abdelatif Koutani,Khalid Elkhader. Stone on the Mesh: Intravesical Erosion after Laparoscopic Promontofixation-A Hidden Cost of Durability. J Clin Med Exp Images. 2026: doi: 10.29328/journal.jcmei.1001040; 10: 006-009
-
Febrile Lumbar Pain Revealing a Massive Collection: Complicated Psoas Abscess Managed SurgicallyMohammed Amine Elafari*,Mamad Ayoub,Mohammed Amine Bibat,Rhayour Anas,Maachi Youssef,Amine Slaoui,Tarik Karmouni,Abdelatif Koutani,Khalid Elkhader. Febrile Lumbar Pain Revealing a Massive Collection: Complicated Psoas Abscess Managed Surgically. J Clin Med Exp Images. 2026: doi: 10.29328/journal.jcmei.1001041; 10: 010-012
-
Feasibility study on the evaluation of the effect of narrow-band CE-Chirp ASSR in the hearing field after hearing aid in hearing-impaired childrenWang Yonghua*,Xing Shuoyao. Feasibility study on the evaluation of the effect of narrow-band CE-Chirp ASSR in the hearing field after hearing aid in hearing-impaired children. Adv Treat ENT Disord. 2019: doi: 10.29328/journal.ated.1001007; 3: 007-011
-
Impact of Community Oriented Ear Care (COEC) on national programme for control of deafness in India: A critical lookSanjeev Davey*,Anuradha Davey,Rajesh Jain. Impact of Community Oriented Ear Care (COEC) on national programme for control of deafness in India: A critical look. Adv Treat ENT Disord. 2020: doi: 10.29328/journal.ated.1001009; 4: 001-002
Most Viewed
-
Effects of dietary supplementation on progression to type 2 diabetes in subjects with prediabetes: a single center randomized double-blind placebo-controlled trialSathit Niramitmahapanya*,Preeyapat Chattieng,Tiersidh Nasomphan,Korbtham Sathirakul. Effects of dietary supplementation on progression to type 2 diabetes in subjects with prediabetes: a single center randomized double-blind placebo-controlled trial. Ann Clin Endocrinol Metabol. 2023 doi: 10.29328/journal.acem.1001026; 7: 00-007
-
Physical Performance in the Overweight/Obesity Children Evaluation and RehabilitationCristina Popescu, Mircea-Sebastian Șerbănescu, Gigi Calin*, Magdalena Rodica Trăistaru. Physical Performance in the Overweight/Obesity Children Evaluation and Rehabilitation. Ann Clin Endocrinol Metabol. 2024 doi: 10.29328/journal.acem.1001030; 8: 004-012
-
Hypercalcaemic Crisis Associated with Hyperthyroidism: A Rare and Challenging PresentationKarthik Baburaj*, Priya Thottiyil Nair, Abeed Hussain, Vimal MV. Hypercalcaemic Crisis Associated with Hyperthyroidism: A Rare and Challenging Presentation. Ann Clin Endocrinol Metabol. 2024 doi: 10.29328/journal.acem.1001029; 8: 001-003
-
Exceptional cancer responders: A zone-to-goDaniel Gandia,Cecilia Suárez*. Exceptional cancer responders: A zone-to-go. Arch Cancer Sci Ther. 2023 doi: 10.29328/journal.acst.1001033; 7: 001-002
-
The benefits of biochemical bone markersSek Aksaranugraha*. The benefits of biochemical bone markers. Int J Bone Marrow Res. 2020 doi: 10.29328/journal.ijbmr.1001013; 3: 027-031

If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."