More Information
Submitted: January 09, 2023 | Approved: February 02, 2023 | Published: February 02, 2023
How to cite this article: Niramitmahapanya S, Chattieng P, Nasomphan T, Sathirakul K. Effects of dietary supplementation on progression to type 2 diabetes in subjects with prediabetes: a single center randomized double-blind placebo-controlled trial. Ann Clin Endocrinol Metabol. 2023; 7: 001-007.
DOI: 10.29328/journal.acem.1001026
Copyright License: © 2023 Niramitmahapanya S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Dietary supplements; Curcumin; Fish oil; Vitamin D; Prediabetes
Effects of dietary supplementation on progression to type 2 diabetes in subjects with prediabetes: a single center randomized double-blind placebo-controlled trial
Sathit Niramitmahapanya1*, Preeyapat Chattieng1, Tiersidh Nasomphan2 and Korbtham Sathirakul2
1Department of Medicine, Rajavithi Hospital, College of Medicine, Rangsit University, Bangkok, Thailand
2Department of Pharmacy, Faculty of Pharmacy, Mahidol University, Bangkok, Thailand
*Address for Correspondence: Sathit Niramitmahapanya, Department of Medicine, Rajavithi Hospital, College of Medicine, Rangsit University, Bangkok, Thailand, Email: [email protected]
Objectives: To examine the effect of dietary supplements on diabetic risk progression, blood glucose level, and lipid profiles.
Methods: A randomized, double-blind, placebo-controlled study was conducted at Rajavithi hospital, Thailand. Participants with prediabetes were randomly allocated to three arms of dietary supplements: placebo (PL) or curcumin plus fish oil and vitamin D (CFD), or curcumin plus fish oil (CF) for 24 weeks. Primary outcomes were the progression of glycemic status and the progression to overt diabetes at 24-week and 36-week follow-ups. Secondary outcomes were changes in glycemic profiles (fasting plasma glucose, 75 g OGTT 2-h plasma glucose or HbA1C), body weight, BMI and lipid profiles.
Results: A total of forty-seven participants (PL, n = 16; CFD, n = 15; CF, n = 16) were included in the study. At the 24-week follow-up, the participants with worsening glycemic status in the intervention groups were lower, CFD, CF and Placebo, 14.29%, 13.33% and 31.25%, respectively. However, the primary outcome, progression of glycemic status, was statistically different, with p - value = 0.046 (p < 0.05) when excluding previous diabetes in the study. As well as the incidence of type 2 diabetes at 24-week follow-up was not statistically different between the three groups, 14.29%, 13.33%, and 12.5%, p - value = 0.699 (p < 0.05) in CFD, CF, PL group, respectively. The secondary outcomes also failed to demonstrate the effect of dietary supplements on blood glucose, lipid profiles, weight, BMI and blood chemistry.
Conclusion: The combined dietary supplements which contained curcumin-fish oil-vitamin D, could lower the glycemic status progression in prediabetes at six months follow-up and were well-tolerated among the participants.
Curcumin, fish oil, and vitamin D have been widely used as dietary supplementation worldwide [1,2]. They are also one of the most popular dietary supplements in Thailand. Curcumin’s pharmacological effect was well known for its anti-inflammatory, and antioxidant properties [3-6]. Moreover, curcumin was found to improve insulin resistance, improve beta cell function, delay the development of type 2 diabetes [7-10] and improve lipid levels in animal models and human studies [11-18].
Fish oil which contained Omega-3 fatty acids was reviewed as improving lipid and blood glucose levels, increasing adiponectin levels, and its anti-inflammatory property, which related to decreased risk of diabetes and cardiovascular events [19-21].
Vitamin D during the COVID era was studied for its anti-inflammatory properties and health benefits of metabolic profile. Vitamin D level in hypertensive patients was related to a 2 times increased risk of cardiovascular events. And the low level of vitamin D level was associated with increased blood glucose levels [22-26].
The combined multiple dietary ingredients in one pill were preferable due to its convenience and multiple health benefits. We were interested in the ingredients which had scientific evidence of lowering blood sugar and lipid level. However, randomized prospective trials on these dietary supplements on health benefits in Thailand were scarce.
The incidence of prediabetes in Thailand was doubling in ten years. In 2011, the prevalence of prediabetes by impaired glucose tolerance (IGT) was 8.7% while in 2021 the prevalence was 15.5% [27]. Approximately 25% of individuals with prediabetes will develop type 2 diabetes in three to five years, and 70% of individuals with prediabetes will develop overt diabetes within their lifetime [28]. Once the person develops prediabetes, the cardiovascular risk has increased, the study found that the cardiovascular events occurred in 18% of individuals with prediabetes compared with 11% of individuals with normal blood glucose levels at five years follow up [29].
Based on health data of Rajavithi hospital healthcare personnel in 2019 - 2020, found that 20% of the staff were at risk of metabolic syndrome such as impaired fasting glucose (IFG), hypercholesterolemia, overweight, and obesity.
This study aimed to evaluate the anti-diabetic property and effect on metabolic profiles of combined dietary supplement regimens, containing curcumin, fish oil and vitamin D which might decrease the risk of diabetes among the healthcare personnel in Rajavithi hospital.
Study design
This study was conducted in Rajavithi Hospital, Bangkok, Thailand. Participants with impaired fasting glucose (IFG) or impaired glucose tolerance (IGT), were recruited from Rajavithi hospital healthcare personnel. They were screened through the yearly routine checkup and laboratory information and were informed by telephone based on the inclusion criteria: Rajavithi hospital healthcare personnel aged more than 20 years old, diagnosed with IFG (impaired fasting glucose); fasting plasma glucose (FPG) 100 mg/dL - 125 mg/dL, IGT (impaired glucose tolerance test); 75 grams oral glucose tolerance test 2 hours plasma glucose (75 g OGTT 2-h PG) 140 mg/dL - 199 mg/dL), or HbA1C 5.7% - 6.5%.
Exclusion criteria included diagnosis with type 2 diabetes, pregnancy, allergic to any ingredients in the study, diagnosis with any medical conditions which need prompt treatment, taking antidiabetic/lipid-lowering agents or any agents that affect blood glucose and lipid level, history of other dietary supplements in previous 3 months before the study, impaired liver function; AST/ALT above 5 times of upper normal range or impaired renal function; GFR < 60 ml/min/1.73 m2.
Intervention
At baseline and at the 24-week visit of the study, the participants were appointed for 75 grams oral glucose tolerance test and other blood tests including hemoglobin A1C, lipid profiles, renal function, liver function, blood cell count (CBC), vitamin D level, serum calcium, phosphate, magnesium, and electrolytes. They were advised to stop eating and drinking 10 – 12 hours before the test. They were asked to drink the liquid that contained glucose 75 grams of within 5 minutes and had blood drawn before and 2 hours after they drank the glucose. At the 12-week visit, the participants were appointed for blood tests including fasting blood glucose, hemoglobin A1C, lipid profiles, renal function, liver function, blood cell count (CBC), vitamin D level serum calcium, phosphate, magnesium, and electrolytes. They were also advised to stop eating and drinking 10 - 12 hours before the blood test.
Curcumin, fish oil, vitamin D combined, and placebo (olive oil) [30-32] soft gels were prepared by Orient innovation company (OIC), Bangkok, Thailand, and were approved by the Thai FDA in March 2020. Each soft gel contained 125 mg of curcumin, 500 mg of fish oil and 100 IU of vitamin D or placebo which were similar in color, smell, taste and texture.
Three regimens of the dietary supplement were provided to the three participants groups, group 1 PL contained a placebo (olive oil), group 2 CFD contained 125 mg of curcumin, 500 mg of fish oil, and 100 IU vitamin D, group 3 CF contained 125 mg of curcumin and 500 mg of fish oil. Participants were appointed every 12 weeks at 0, 12 and 24 weeks of intervention. Participants took 1 capsule twice a day.
The amount of each ingredient was under the limit of the Thai FDA announcement for active ingredients allowed in food supplement products. Curcumin should not be more than 2,000 grams per day. The fish oil should meet the quality and amount of not more than 3 grams allowed in the Thai FDA announcement of fish oil in 2021. Vitamin D should not be more than 400 IU per day in the dietary supplementation and not lower than 15% of the Thai recommended dietary allowance (Thai RDA). We decided to use our dietary supplement dosage of up to 10% - 50% of the maximum dosage of each ingredient in this study due to safety concerns.
The quality-of-life scores [33], compliance assessment, and adverse effects were monitored by phone call, SMS, or chat application monthly due to the social distancing policy during a COVID outbreak in Thailand. This study was approved by Ethics Committees on Researches Involving Human Subjects Rajavithi Hospital, Bangkok, Thailand in April 2021, EC number 081/2021. The trial has been registered with Rajavithi clinical trial registry in May 2021.
Procedures
All participants gave their informed consent before participating in the study. The three groups visited 4 times at 0, 12, 24 and 36 weeks of intervention. Personal demographic information was obtained by case record form and phone calls for additional data. At each visit, blood pressure, height, weight, BMI were measured and 10 ml - 15 ml blood samples were collected after 10 - 12 hours of fasting and 75 grams glucose tolerance test was performed at weeks 0 and 24. The short form of compliance and quality of life assessment in Thai, provided in the Supplementary Appendix, were obtained at 0, 24 weeks of the study.
Study outcomes
Primary outcomes were the progression of glycemic status and the incidence of type 2 diabetes at 24-week and 36-week follow-ups. The progression of glycemic status was estimated by glycemic status progression in Table 1. The 36-week follow-up outcome was ongoing and will be finished in April 2022.
| Table 1: Glycemic status classification and definition of prediabetes [34]. | ||
| Glycemic status classification | Definition | |
| 1 | Normal | FPG < 100 mg/dL and HbA1C < 5.7% |
| 2 | IFG | FPG 100 mg/dL - 125 mg/dL or HbA1C 5.7 – 6.4% |
| 3 | IGT | 75-g OGTT 2 hr. PG 140 mg/dL - 199 mg/dL or HbA1C 5.7% – 6.4% |
| 4 | IFG and IGT | FPG 100 mg/dL - 125 mg/dL and 75-g OGTT 2 hr. PG 140 mg/dL - 199 mg/dL |
| 5 | T2D by 75-g OGTT 2 hr. PG or HbA1C |
75-g OGTT 2 hr. PG ≥ 200 mg/dL or HbA1C ≥ 6.5% |
| 6 | T2D by 75-g OGTT 2 hr. PG and HbA1C |
75-g OGTT 2 hr. PG ≥ 200 mg/dL and HbA1C ≥ 6.5% |
| FPG: Fasting Plasma Glucose; IFG: Impaired Fasting Glucose; IGT: Impaired Glucose Tolerance; 75-g OGTT: 75 Grams Oral Glucose Tolerance Test; 2-h PG: 2 hours Plasma Glucose. *For all three tests, the risk is continuous, extending below the lower limit of the range and becoming disproportionately greater at the higher end of the range. |
||
The glycemic status progression was clinically important due to the different annual rates of type 2 diabetes progression in each glycemic status, Table 2.
| Table 2: The annual rates of progression to type 2 diabetes [37]. | ||||
| Forms of glucose tolerance | IFG | IGT | IFG + IGT | T2D |
| Normal glucose tolerance | 1.3 | 3.9 | 0.5 | 0.6 |
| IFG | 3.7 | 6.5 | 2.4 | |
| IGT | 0.9 | 2.7 | ||
| IFG + IGT | 9.9 | |||
| IFG: Impaired Fasting Glucose; IGT: Impaired Glucose Tolerance; T2D: Type 2 Diabetes | ||||
Secondary outcomes of this study were changes in glycemic profiles (fasting plasma glucose, 75 g OGTT 2-h plasma glucose or HbA1C), body weight, Body Mass Index (BMI) and lipid profiles at 24, 36 weeks follow-up.
Randomization and statistical analysis
Randomization was conducted using simple random sampling. None of the participants, technicians, statisticians, or investigators know the prescribed regimen during the study. Sample size [35] was calculated based on data from the study by Chuengsamarn, et al. [7], n = 15 per study arm was estimated. We included a total of 47 participants in the study. Baseline characteristics were analyzed by using an independent sample t - test for continuous data and a chi-square test for categorical data. The primary and secondary outcomes were analyzed by paired sample t - test.
Ethical considerations
The study was approved by the Rajavithi human research study ethics committees, Rajavithi hospital in April 2021. The trial has been registered with Rajavithi clinical trial registry. We obtained informed consent from all participants.
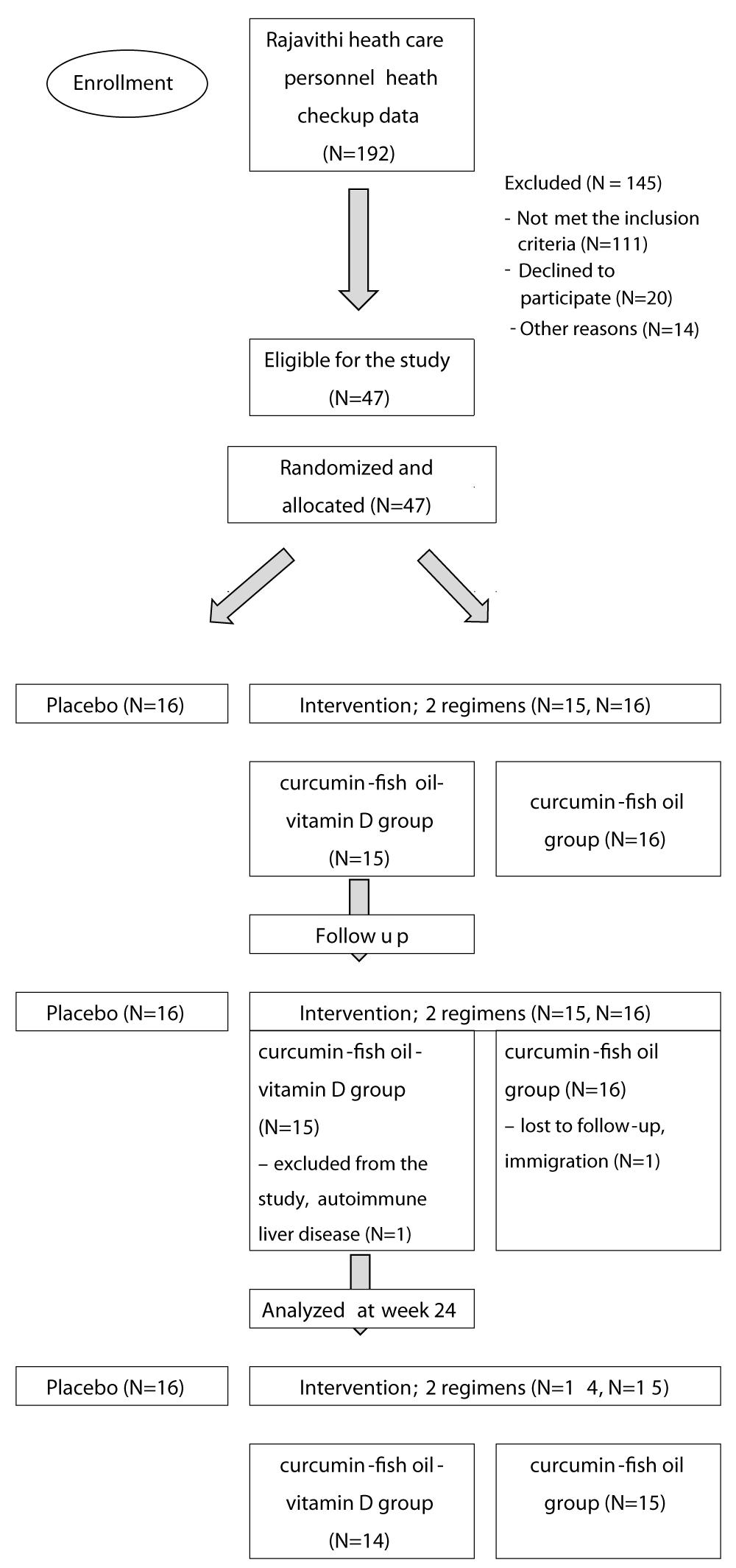
The study started on July 2021 to February 2022 in Rajavithi Hospital, Bangkok, Thailand. 192 health checkup data was reviewed and selected. 47 participants were eligible and randomized to three groups (N = 47) (Figure 1). One participant in the curcumin-fish oil-vitamin D group was withdrawn from the study at week 8 due to abnormal liver function test and was diagnosed with autoimmune hepatitis by liver biopsy. One participant in the curcumin-fish oil group was lost to follow-up due to a move to another province. At the end of the study, 14 participants in the curcumin-fish oil-vitamin D group, 15 in the curcumin-fish oil group and 16 in the placebo group completed the study. The baseline characteristics between the intervention groups and placebo group were not statistically different as shown in Table 3. The adherence of the participants was more than 80%. The quality-of-life score at baseline and at the 24-week outcome in each group was not different, Table S3 in the Supplementary Appendix. In the outcomes of 36 weeks, we found only a curcumin base regimen that decreases the risk of diabetes progression in 24 weeks study but not found in 36 weeks study.
Figure 1: Consort flow diagram.
| Table 3: Baseline characteristics | ||||
| Variables | Group 1 PL (N = 16) | Group 2 CFD (N = 15) | Group 3 CF (N = 16) | p - value |
| Sex (%) (male: female) Male (%) Female (%) |
2:14 12.5 87.5 |
0:15 0 100 |
2:14 12.5 87.5 |
0.359 |
| Age (years) | 50 ± 8.76 | 54.6 ± 10.11 | 46.75 ± 9.67 | 0.082 |
| BW (kg) | 69.52 ± 13.60 | 70.41 ± 19.79 | 73.54 ± 15.50 | 0.769 |
| BMI (kg/m2) | 27.45 ± 5.16 | 29.22 ± 7.40 | 28.8 ± 4.77 | 0.677 |
| FPG (mg/dL) | 102.56 ± 12.09 | 97.47 ± 11.65 | 100.44 ± 10.61 | 0.469 |
| 75-g OGTT PG at 2 hr. (mg/dL) | 148.73 ± 57.37 | 132.93 ± 39.57 | 121.06 ± 32.3 | 0.228 |
| HbA1C (%) | 5.89 ± 0.39 | 5.83 ± 0.46 | 5.72 ± 0.38 | 0.503 |
| LDL (mg/dL) | 144.94 ± 40.78 | 126.60 ± 46.91 | 128.25 ± 30.58 | 0.368 |
| Triglycerides (mg/dL) | 132.94 ± 85.36 | 162.20 ± 127.46 | 132.19 ± 54.61 | 0.600 |
| Total cholesterol (mg/dL) | 216.69 ± 45.89 | 209.14 ± 51.96 | 200.19 ± 32.61 | 0.571 |
| HDL (mg/dL) | 53.88 ± 10.21 | 60.47 ± 16.60 | 52.88 ± 10.91 | 0.215 |
| Creatinine (mg/dL) | 0.69 ± 0.16 | 0.66 ± 0.12 | 0.70 ± 0.15 | 0.701 |
| AST (U/L) | 26.25 ± 13.33 | 24.20 ± 8.36 | 22.19 ± 11.61 | 0.602 |
| ALT (U/L) | 34.81 ± 31.49 | 25.33 ± 15.21 | 22.81 ± 13.27 | 0.269 |
| Vitamin D level (ng/ml) | 23.99 ± 6.40 | 24.23 ± 10.56 | 22.14 ± 6.77 | 0.751 |
| Hb (g/dL) | 13.5 ± 0.92 | 13.44 ± 0.87 | 13.56 ± 1.18 | 0.957 |
| PL: Placebo Group; CFD: Curcumin-Fish oil-vitamin D group; CF: Curcumin-Fish oil group; FPG: Fasting Plasma Glucose; OGTT: 75-gram Oral Glucose Tolerance Test; A1C: Hemoglobin A1C; LDL: LDL cholesterol; TG: Triglycerides. TC: Total Cholesterol; HDL: HDL Cholesterol; Cr: Creatinine; AST: Aspartate Transaminase; ALT: Alanine Transaminase; VitD: Vitamin D level; Hb: Hemoglobin; BW: Body Weight (kg); BMI: Body Mass Index. |
||||
We found that the glycemic status at 24-week follow-up in the two intervention groups tended to be better than the placebo group. The numbers of the participants with worsening glycemic status at 24-week follow-up in the intervention groups were lower than in the placebo group, as shown in Table 4 and (Supplementary Appendix S1-S3), CFD, CF, Placebo, 14.29%, 13.33%, 31.25%, respectively. However, the progression of glycemic status at 24-week follow-up was not statistically different between the three groups, p - value = 0.311 (p < 0.05) due to the small number of participants in the study. As well as the incidence of type 2 diabetes at 24-week follow-up was not different between the three groups, 14.29%, 13.33% and 12.5%, p - value = 0.699 (p < 0.05) in CFD, CF, and PL group, respectively. Moreover, the means of body weight, BMI change or blood chemistries used in the study, such as FPG, 75 gm OGTT 2-h PG, HbA1C, and Lipid profiles, as shown in Table 5, were not statistically different. No serious adverse events were reported. In Table 5, we found that in curcumin base treatment in group 2(CFD) and group 3(CF) trend of FBS and triglyceride levels that lower than in the control group. We found greater changes from baseline in both intervention groups, but not statistically significant.
| Table 5: Secondary outcomes: changes of parameters from baseline (Table S1 and S2 appendix). | ||||
| Variables | Group 1 PL (N = 16) |
Group 2 CFD (N = 14) |
Group 3 CF (N = 15) |
p - value |
| BW (kg) | -0.84 ± 3.05 | 0.39 ± 2.01 | 0.15 ± 1.75 | 0.321 |
| BMI (kg/m2) | -0.35 ± 1.22 | 0.15 ± 0.84 | 0.05 ± 0.70 | 0.318 |
| FPG (mg/dL) | 0.75 ± 9.56 | -2.57 ± 4.82 | -1.67 ± 13.88 | 0.652 |
| 75-g OGTT PG at 2 hr. (mg/dL) | -6.25 ± 68.74 | -10.71 ± 20.46 | -2.8 ± 36.24 | 0.904 |
| HbA1C (%) | 0.02 ± 0.25 | 0.08 ± 0.25 | 0.15 ± 0.24 | 0.362 |
| LDL (mg/dL) | -5.63 ± 28.27 | -5.07 ± 50 | -8.27 ± 30.88 | 0.969 |
| Triglycerides (mg/dL) | -2.00 ± 53.84 | -37.07 ± 75.79 | -1.60 ± 39.12 | 0.177 |
| Total cholesterol (mg/dL) | -7.13 ± 26.90 | -8.38 ± 40.17 | -17.80 ± 39.21 | 0.670 |
| HDL (mg/dL) | -0.75 ± 4.54 | -0.50 ± 6.30 | -2.27 ± 6.12 | 0.658 |
| Creatinine (mg/dL) | -0.01 ± 0.09 | 0.04 ± 0.08 | -0.02 ± 0.06 | 0.140 |
| AST (U/L) | -3.40 ± 6.84 | -2.64 ± 5.67 | 1.00 ± 15.72 | 0.480 |
| ALT (U/L) | -6.20 ± 16.21 | -0.71 ± 8.48 | 1.67 ± 18.21 | 0.349 |
| Vitamin D level (ng/ml) | -1.51 ± 4.32 | 2.32 ± 5.28 | -0.95 ± 4.37 | 0.098 |
| Hb (g/dL) | -0.1 ± 0.44 | -0.45 ± 0.61 | -0.51 ± 0.76 | 0.180 |
| PL: Placebo Group; CFD: Curcumin-Fish Oil-Vitamin D group; CF: Curcumin-Fish oil group; FPG: Fasting Plasma Glucose; OGTT: 75-gram Oral Glucose Tolerance Test; A1C: Hemoglobin A1C; LDL: LDL cholesterol; TG: Triglycerides; TC: Total Cholesterol; HDL: HDL cholesterol; Cr: Creatinine; AST: Aspartate Transaminase; ALT: Alanine Transaminase; VitD: Vitamin D level; Hb: Hemoglobin; BW: Body Weight (kg); BMI: Body Mass Index. |
||||
Only group 2 that composite of Vitamin D in a regimen that the only regimen that showed an increased level of vitamin D higher than another group with a p - value of 0.098 as shown in Table 5.
In an analysis of the final report, we didn’t find statistically significantly different in glycemic parameters and any diabetes risk progression score to T2D (not report).
Adverse effects
During the study, we monitored the adverse effects of the dietary supplements [36] by phone call, weight, blood pressure, and blood chemistries for liver and kidney functions. We found no significant differences in the interventions and placebo groups. A few participants in the intervention groups reported minor nonspecific symptoms, such as an itching symptom that occurred in one participant in the first 2 weeks of the study and was spontaneously resolved without any treatment. GERD-like symptom sometimes occurred in two participants and was improved with the proton pump inhibitors and prokinetic medications. These symptoms were remarked as not related to the prescribed dietary supplements. During the 24-week study, none of the participants developed cardiovascular events or death.
This study aimed to examine the health benefit of metabolic profiles of combined dietary supplements, which contained curcumin, fish oil and vitamin D. The results represented the clinical benefit of the decreased risk of diabetic progression among prediabetes individuals. The effect of the dietary supplements on body weight, BMI change, or blood chemistries, such as FPG, 75 gm OGTT 2-hr PG, HbA1C, and lipid profiles was not shown a significant difference compared with placebo.
The previous studies [7,8] showed the favorable effects of curcumin, one of the ingredients in our combined dietary supplements, with higher dosages of up to 1,500 mg of the curcumin daily prescribed on body weight different from our study lower than 6 times dose of curcumin a day (curcumin 250 mg daily in our study), FPG and HbA1C changes.
The curcumin particle process in this study that made by a novel innovation technique with spray dries which made curcumin particle-sized that lower to nanoparticle by 0.4 nm ± 0.02 nm. With a Z-average of the particle around 166.6 nm ± 2.34 nm. The composite of Tuna fish oil could be the proper vector that takes curcumin particles to the cell directly independent of another process of distribution into the cell membrane of target organs such as another commercial turmeric extract powder.
Moreover, in our study, the participants were not aggressive and strictly adhered to dietary and lifestyle modifications. During the COVID outbreak lockdown, most participants were less active and increased amount of food intake, the information obtained from participant interviewing during the follow-up. Our study outcomes also showed weight gain in both intervention groups, 0.39 ± 2.01, 0.15 ± 1.75 kg, but weight loss in the placebo group, -0.84 ± 3.05 kg, p - value = 0.321 (p < 0.05). Two participants were the high-risk contact with COVID patients and were home-quarantined for fourteen days, however, none of the participants was infected with COVID-19 during the study.
This study was conducted in a single center and included only healthcare personnel in the study due to the COVID lockdown policy which did not represent the generalizability of the population and include a too small number of participants. A 12-week follow-up and adherence evaluation by phone call during the COVID era was quite a long duration which may lead to poorer adherence compared with short-duration, face-to-face reminders. Daily calorie intake control between the three groups was not implemented, while the previous studies input the dietary and lifestyle modification along with dietary supplementation [7,8]. Curcumin dosage in this study might be insufficient or underdosed compared with the previous studies.
Larger numbers, more variety of participants, and longer periods of the study including different dosages of dietary ingredients were needed in further studies to support the study outcomes. And the two intervention groups which included curcumin and fish oil with and without vitamin D respectively can easily use in further studies for increasing the dosage of curcumin and fish oil without overdosage of vitamin D allowed.
In conclusion, the combined dietary supplements containing curcumin-fish oil-vitamin D benefit beyond the progression of glycemic status, prevent the risk of prediabetes progression to overt diabetes, and its minor adverse effect is well-tolerated in most people. Current evidence suggests that carbohydrate, lipid and amino acid metabolites not only are altered in individuals but also exhibit significant prospective associations with prediabetes and/or type 2 diabetes. The basic nutrition for pre-diabetes should be concerned with low carbohydrate components, sugar from food begins to build up in your bloodstream because insulin can’t easily move the sugar from the extracellular fluid. Curcumin and fish oil with/without vitamin D supplementation may decrease progression to diabetes but should be integrated with exercise according to the standard recommendation.
Funding and resources
This trial was funded by Rajavithi Hospital, Department of Medical Services, Ministry of Public health, Thailand.
- Hamulka J, Jeruszka-Bielak M, Górnicka M, Drywień ME, Zielinska-Pukos MA. Dietary Supplements during COVID-19 Outbreak. Results of Google Trends Analysis Supported by PLifeCOVID-19 Online Studies. Nutrients. 2020 Dec 27;13(1):54. doi: 10.3390/nu13010054. PMID: 33375422; PMCID: PMC7823317.
- Kamiński M, Kręgielska-Narożna M, Bogdański P. Determination of the Popularity of Dietary Supplements Using Google Search Rankings. Nutrients. 2020 Mar 26;12(4):908. doi: 10.3390/nu12040908. PMID: 32224928; PMCID: PMC7231191.
- Miquel J, Bernd A, Sempere JM, Díaz-Alperi J, Ramírez A. The curcuma antioxidants: pharmacological effects and prospects for future clinical use. A review. Arch Gerontol Geriatr. 2002 Feb;34(1):37-46. doi: 10.1016/s0167-4943(01)00194-7. PMID: 14764309.
- Sundar Dhilip Kumar S, Houreld NN, Abrahamse H. Therapeutic Potential and Recent Advances of Curcumin in the Treatment of Aging-Associated Diseases. Molecules. 2018 Apr 5;23(4):835. doi: 10.3390/molecules23040835. PMID: 29621160; PMCID: PMC6017430.
- Asadi S, Gholami MS, Siassi F, Qorbani M, Khamoshian K, Sotoudeh G. Nano curcumin supplementation reduced the severity of diabetic sensorimotor polyneuropathy in patients with type 2 diabetes mellitus: A randomized double-blind placebo- controlled clinical trial. Complement Ther Med. 2019 Apr;43:253-260. doi: 10.1016/j.ctim.2019.02.014. Epub 2019 Feb 28. PMID: 30935539.
- Djalali M, Abdolahi M, Hosseini R, Miraghajani M, Mohammadi H, Djalali M. The effects of nano-curcumin supplementation on Th1/Th17 balance in migraine patients: A randomized controlled clinical trial. Complement Ther Clin Pract. 2020 Nov;41:101256. doi: 10.1016/j.ctcp.2020.101256. Epub 2020 Oct 29. PMID: 33147541.
- Chuengsamarn S, Rattanamongkolgul S, Luechapudiporn R, Phisalaphong C, Jirawatnotai S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care. 2012 Nov;35(11):2121-7. doi: 10.2337/dc12-0116. Epub 2012 Jul 6. PMID: 22773702; PMCID: PMC3476912.
- Chuengsamarn S, Rattanamongkolgul S, Phonrat B, Tungtrongchitr R, Jirawatnotai S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: a randomized controlled trial. J Nutr Biochem. 2014 Feb;25(2):144-50. doi: 10.1016/j.jnutbio.2013.09.013. Epub 2013 Nov 6. PMID: 24445038.
- Thota RN, Acharya SH, Garg ML. Curcumin and/or omega-3 polyunsaturated fatty acids supplementation reduces insulin resistance and blood lipids in individuals with high risk of type 2 diabetes: a randomised controlled trial. Lipids Health Dis. 2019 Jan 26;18(1):31. doi: 10.1186/s12944-019-0967-x. PMID: 30684965; PMCID: PMC6347796.
- Heshmati J, Moini A, Sepidarkish M, Morvaridzadeh M, Salehi M, Palmowski A, Mojtahedi MF, Shidfar F. Effects of curcumin supplementation on blood glucose, insulin resistance and androgens in patients with polycystic ovary syndrome: A randomized double-blind placebo-controlled clinical trial. Phytomedicine. 2021 Jan;80:153395. doi: 10.1016/j.phymed.2020.153395. Epub 2020 Oct 22. PMID: 33137599.
- Ramírez-Tortosa MC, Mesa MD, Aguilera MC, Quiles JL, Baró L, Ramirez-Tortosa CL, Martinez-Victoria E, Gil A. Oral administration of a turmeric extract inhibits LDL oxidation and has hypocholesterolemic effects in rabbits with experimental atherosclerosis. Atherosclerosis. 1999 Dec;147(2):371-8. doi: 10.1016/s0021-9150(99)00207-5. PMID: 10559523.
- Manjunatha H, Srinivasan K. Hypolipidemic and antioxidant effects of dietary curcumin and capsaicin in induced hypercholesterolemic rats. Lipids. 2007 Dec;42(12):1133-42. doi: 10.1007/s11745-007-3120-y. Epub 2007 Oct 25. PMID: 17960446.
- Alwi I, Santoso T, Suyono S, Sutrisna B, Suyatna FD, Kresno SB, Ernie S. The effect of curcumin on lipid level in patients with acute coronary syndrome. Acta Med Indones. 2008 Oct;40(4):201-10. PMID: 19151449.
- Hussein S, El-senosi Y, Ragab M, Hammad M. Hypolipidemic effect of curcumin in hyper-cholesterolemic rats. Benha Veterinary Medical Journal. 2014 Dec;27(2):277-89.
- Yang YS, Su YF, Yang HW, Lee YH, Chou JI, Ueng KC. Lipid-lowering effects of curcumin in patients with metabolic syndrome: a randomized, double-blind, placebo-controlled trial. Phytother Res. 2014 Dec;28(12):1770-7. doi: 10.1002/ptr.5197. Epub 2014 Aug 6. PMID: 25131839.
- Tang Y. Curcumin targets multiple pathways to halt hepatic stellate cell activation: updated mechanisms in vitro and in vivo. Dig Dis Sci. 2015 Jun;60(6):1554-64. doi: 10.1007/s10620-014-3487-6. Epub 2014 Dec 23. PMID: 25532502.
- Morrone Mda S, Schnorr CE, Behr GA, Gasparotto J, Bortolin RC, da Boit Martinello K, Saldanha Henkin B, Rabello TK, Zanotto-Filho A, Gelain DP, Moreira JC. Curcumin Supplementation Decreases Intestinal Adiposity Accumulation, Serum Cholesterol Alterations, and Oxidative Stress in Ovariectomized Rats. Oxid Med Cell Longev. 2016;2016:5719291. doi: 10.1155/2016/5719291. Epub 2015 Nov 23. PMID: 26640615; PMCID: PMC4658407.
- Qin S, Huang L, Gong J, Shen S, Huang J, Ren H, Hu H. Efficacy and safety of turmeric and curcumin in lowering blood lipid levels in patients with cardiovascular risk factors: a meta-analysis of randomized controlled trials. Nutr J. 2017 Oct 11;16(1):68. doi: 10.1186/s12937-017-0293-y. PMID: 29020971; PMCID: PMC5637251.
- Brinson BE, Miller S. Fish oil: what is the role in cardiovascular health? J Pharm Pract. 2012 Feb;25(1):69-74. doi: 10.1177/0897190011406983. PMID: 21676848.
- Mozaffarian D, Wu JH, de Oliveira Otto MC, Sandesara CM, Metcalf RG, Latini R, Libby P, Lombardi F, O'Gara PT, Page RL, Silletta MG, Tavazzi L, Marchioli R. Fish oil and post-operative atrial fibrillation: a meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2013 May 28;61(21):2194-6. doi: 10.1016/j.jacc.2013.02.045. Epub 2013 Mar 26. PMID: 23541970; PMCID: PMC3697850.
- Villani AM, Crotty M, Cleland LG, James MJ, Fraser RJ, Cobiac L, Miller MD. Fish oil administration in older adults with cardiovascular disease or cardiovascular risk factors: is there potential for adverse events? A systematic review of the literature. Int J Cardiol. 2013 Oct 9;168(4):4371-5. doi: 10.1016/j.ijcard.2013.05.054. Epub 2013 Jun 3. PMID: 23742929.
- Napartivaumnuay N, Niramitmahapanya S, Deerochanawong C, Suthornthepavarakul T, Sarinnapakorn V, Jaruyawongs P. Maternal 25 hydroxyvitamin D level and its correlation in Thai gestational diabetes patients. J Med Assoc Thai. 2013 Mar;96 Suppl 3:S69-76. PMID: 23682526.
- Mirhosseini N, Vatanparast H, Mazidi M, Kimball SM. The Effect of Improved Serum 25-Hydroxyvitamin D Status on Glycemic Control in Diabetic Patients: A Meta-Analysis. J Clin Endocrinol Metab. 2017 Sep 1;102(9):3097-3110. doi: 10.1210/jc.2017-01024. PMID: 28957454.
- Niroomand M, Fotouhi A, Irannejad N, Hosseinpanah F. Does high-dose vitamin D supplementation impact insulin resistance and risk of development of diabetes in patients with pre-diabetes? A double-blind randomized clinical trial. Diabetes Res Clin Pract. 2019 Feb;148:1-9. doi: 10.1016/j.diabres.2018.12.008. Epub 2018 Dec 21. PMID: 30583032.
- Mohammadi S, Hajhashemy Z, Saneei P. Serum vitamin D levels in relation to type-2 diabetes and prediabetes in adults: a systematic review and dose-response meta-analysis of epidemiologic studies. Crit Rev Food Sci Nutr. 2022;62(29):8178-8198. doi: 10.1080/10408398.2021.1926220. Epub 2021 Jun 2. PMID: 34076544.
- Hahn J, Cook NR, Alexander EK, Friedman S, Walter J, Bubes V, Kotler G, Lee IM, Manson JE, Costenbader KH. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ. 2022 Jan 26;376:e066452. doi: 10.1136/bmj-2021-066452. PMID: 35082139; PMCID: PMC8791065.
- The International Diabetes Federation (IDF). Diabetes atlas: Thailand diabetes report 2000 – 2045. URL:https://diabetesatlas.org/data/en/country/196/th.html.
- Hostalek U. Global epidemiology of prediabetes - present and future perspectives. Clin Diabetes Endocrinol. 2019 May 9;5:5. doi: 10.1186/s40842-019-0080-0. PMID: 31086677; PMCID: PMC6507173.
- Mando R, Waheed M, Michel A, Karabon P, Halalau A. Prediabetes as a risk factor for major adverse cardiovascular events. Ann Med. 2021 Dec;53(1):2090-2098. doi: 10.1080/07853890.2021.2000633. PMID: 34761971; PMCID: PMC8592612.
- Schwingshackl L, Hoffmann G. Monounsaturated fatty acids, olive oil and health status: a systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014 Oct 1;13:154. doi: 10.1186/1476-511X-13-154. PMID: 25274026; PMCID: PMC4198773.
- Schwingshackl L, Lampousi AM, Portillo MP, Romaguera D, Hoffmann G, Boeing H. Olive oil in the prevention and management of type 2 diabetes mellitus: a systematic review and meta-analysis of cohort studies and intervention trials. Nutr Diabetes. 2017 Apr 10;7(4):e262. doi: 10.1038/nutd.2017.12. PMID: 28394365; PMCID: PMC5436092.
- Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pintó X, Basora J, Muñoz MA, Sorlí JV, Martínez JA, Fitó M, Gea A, Hernán MA, Martínez-González MA; PREDIMED Study Investigators. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med. 2018 Jun 21;378(25):e34. doi: 10.1056/NEJMoa1800389. Epub 2018 Jun 13. PMID: 29897866.
- Mahanniran S, Tantipiwattanasakul W, Poompaisarnchai W. 2002. WHO: Quality of life questionnaire, SF-36 (WHOQOL)-BREF, Thai version. https://www.dmh.go.th/test/whoqol/, accessed 1 June 2021.
- American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022 Jan 1;45(Suppl 1):S17-S38. doi: 10.2337/dc22-S002. PMID: 34964875.
- Bernard R. Fundamentals of biostatistics. 5th ed. Massachusetts: Duxbury Press; 2000.
- Saokaew S, Suwankesawong W, Permsuwan U, Chaiyakunapruk N. Safety of herbal products in Thailand: an analysis of reports in the thai health product vigilance center database from 2000 to 2008. Drug Saf. 2011 Apr 1;34(4):339-50. doi: 10.2165/11586590-000000000-00000. PMID: 21417506.
- Kohler C, Henkel E, Temelkova-Kurktschiev T, Fuecker K, Hanefeld M. Incidence of impaired fasting glucose tolerance and type 2 diabetes in German risk population: the RIAD study. Diabetologia. 2001; 44: A108.