More Information
Submitted: March 26, 2021 | Approved: April 06, 2021 | Published: April 07, 2021
How to cite this article: Capdevila L, Borràs A, Berlanga E, Sánchez- Manubens J, Rivera J, et al. Usefulness of salivary cortisol as a marker of secondary adrenal insufficiency in paediatric patients. Ann Clin Endocrinol Metabol. 2021; 5: 024-028.
DOI: 10.29328/journal.acem.1001020
ORCiD: orcid.org/0000-0003-3344-8269
Copyright License: © 2021 Capdevila L, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Salivary cortisol; Adrenal insufficiency; Children
Abbreviations: AI: Adrenal Insufficiency; PC: Plasma Cortisol; ACTH: Adrenocorticotropic Hormone; SC: Salivary Cortisol
Usefulness of salivary cortisol as a marker of secondary adrenal insufficiency in paediatric patients
Laura Capdevila1, Ariadna Borràs1, Eugenio Berlanga1, Judith Sánchez- Manubens2, Josefa Rivera2 and Raquel Corripio2*
1Paediatric Endocrine Department, Laboratory Department. UDIAT, Parc Taulí Sabadell, Sabadell Hospital, Autonomous University of Barcelona (UAB), Sabadell, Spain
2Paediatric Rheumatology Department, Parc Taulí Sabadell, Sabadell Hospital, Autonomous University of Barcelona (UAB), Sabadell, Spain
*Address for Correspondence: Dr. Raquel Corripio, Paediatric Endocrine Department, Parc Taulí Sabadell, Sabadell Hospital, Autonomous University of Barcelona (UAB), Sabadell, Spain, C/ Parc Taulí S/N, 08208, Sabadell, Spain, Tel: +34937458269; Fax: +34937160646; Email: [email protected]
Background: The main cause of adrenal insufficiency (AI) in paediatric patients is prolonged treatment with corticosteroids. Determination of plasma cortisol (PC) during ACTH test is the most used adrenal function indicator in clinical practice. However, determination of salivary cortisol (SC), a simple test especially useful in children in order to avoid invasive procedures, can be used as an alternative technique for the diagnosis of adrenal disease.
Methods: A two-year prospective study (January 2014-January 2016) in paediatric patients (2-18 years of age) treated with corticosteroids for more than fifteen days, who were investigated for suspected AI. Low-dose ACTH test was used to determine adrenal function and samples for SC and PC were obtained simultaneously in basal situation and during the test (at 30, 60 and 90 minutes).
Results: 230 samples (118 PC-112 SC) of 30 studies belonging to 20 patients (4 males), mean age 10.93 years ± 3.69 SD. Pearson’s correlation coefficient showed a positive correlation between PC and SC (r = 0.618, p < 0.001). All the studies with some determination of PC higher than 18 μg/dL (n = 8) had a SC peak higher than 0.61 μg/dL with a specificity of 66.67% and a sensitivity of 93.94% (ROC analysis).
Conclusion: Measurement of SC is a less invasive, easier and quicker test than PC to measure plasma free cortisol levels. In our study, a SC peak in low-dose ACTH test higher than 0.61 μg/dL was able to discriminate patients without AI, and proved to be a useful tool in the initial evaluation of children with suspected AI.
The activation of the hypothalamic-pituitary-adrenal axis in response to critical illness and the resulting release of cortisol from the adrenal cortex are essential to stress adaptation. Adrenal insufficiency (AI) is described as the inability of adrenal glands to produce an appropriate hormonal secretion not only under stress but also in basal situation. Therefore, a low baseline plasma cortisol (PC) (< 5 μg/dL) and a poor cortisol response to stimulation with exogenous adrenocorticotropic hormone (peak < 18 μg/dL) are some of the defining criteria of this condition [1,2]. It is well known that the main cause of AI in paediatric patients is prolonged treatment with exogenous corticosteroids, which is an iatrogenic cause derived from the increasing complexity of paediatric pathologies and the increased use of prolonged high-dose corticosteroid therapy.
In clinical practice, adrenal function is usually assessed by the total PC (determined by low-dose ACTH test). This implies the placement of a vascular access which is often a traumatic experience for children.
PC includes protein-bound fraction and serum-free cortisol. The latter constitutes the biologically active form of the hormone and is responsible for glucocorticoid activity on peripheral organs. Most of the circulating cortisol is bound to plasma proteins (over 90%), such as cortisol-binding globulin (CBG) and albumin, whereas only about 10% of circulating cortisol is free. Hence, the measurement of plasma-free cortisol level has been considered more representative of adrenal function (especially in critically ill adults and children) [1,2], because some conditions, such as hypoalbuminaemia or hypoproteinaemia (frequent in critically ill patients or in patients with cirrhosis), may lead to misinterpretation of adrenal function with an overestimation of the prevalence of AI. But the direct measurement of free PC is a laboratory-dependent and time-consuming procedure that is not available for routine use. Salivary cortisol (SC) is one of the several indirect methods available to determine free PC [3], as SC levels accurately reflect free PC [4] even in cases of hypoalbuminaemia or CBG abnormality [1,5]. For this reason, in the last years, this technique (SC) has been introduced as a non-invasive tool in the diagnosis of adrenal cortical disorders, for its simplicity and applicability in the paediatric population. However, few studies to date have evaluated the usefulness of SC as a diagnostic method in children with AI. No interactions between exogenous corticoids and SC have been described [6].
The aim of the present study was to assess the usefulness of determining salivary cortisol levels as a diagnostic tool in children with suspected secondary iatrogenic AI.
The study was carried out at the outpatient Paediatric Endocrine Department of Sabadell Hospital. We designed a prospective 2-year study, from January 2014 to January 2016, in paediatric patients (aged between 2 and 18 years). Inclusion criterion was: children with suspected AI secondary to prolonged corticosteroid therapy (over 15 days), received as a treatment for various underlying pathologies. These patients were referred to the Paediatric Endocrinology Unit from any paediatric section of our hospital. Written informed consent was obtained from all patients’ parents and the investigation was conducted according to the Declaration of Helsinki. The study was approved by the Ethics Committee of our Institution (reference code CEIC 2014510).
Samples of twenty children (4 male), median age 10.93 years ± 3.69 SD years were evaluated. All the patients had received long-term steroid treatment for various disorders (mainly rheumatic diseases, such as systemic lupus erythematosus, juvenile dermatomyositis, and others). After informed consent was obtained, clinical data (age, gender, ethnicity, anthropometric data, Cushing features and main pathology) were recorded. In a second visit, plasma and salivary cortisol levels after ACTH test was performed at 8:00 - 9:00 am, after fasting for at least 8 hours, 1 hour after waking up [6], 24-48 hours after steroid withdrawal, always in the outpatient day hospital and performed by same nurse [7] to avoid variability. A peripheral vascular catheter was placed and blood sample for PC baseline levels was collected. After careful mouth cleansing, a specific device was used to collect a saliva sample (Sarstedt Cortisol Salivette® Device). According to the supplier’s instructions, the sponge is placed directly into the mouth and patients gently chew and roll it around for 2-3 minutes before spitting it back into the tube and the sample can be collected. After collecting baseline plasma and saliva samples, ACTH stimulation test was performed using a dose of 1µg synthetic adrenocorticotropic hormone, administered by intramuscular injection (Synacthen®). Blood samples for plasma cortisol were collected at 30, 60 and 90 minutes; saliva samples were collected simultaneously. These saliva and plasma samples were subjected to electrochemiluminescence (E-170, Roche Diagnostics). Detection limit: 0.018 µg/dL. Coefficient of variation (CV): 6.1% intraday at concentration of 0.17µg/dL; 11.5% interday at concentration of 0.292 µg/dL.
Data are expressed as mean ± SD for quantitative variables and as percentages for categorical variables unless otherwise indicated. Pearson’s linear regression analysis was used to analyze the correlation between plasma and saliva cortisol, while the ROC curve was used to determine cut-off for SC. A p value < 0.05 was considered as significant. All analyses were performed with SPSS 21.0 version (SPSS, Chicago, USA).
A total of 230 samples were analyzed (118 of plasma cortisol and 112 of salivary cortisol), of 30 studies (consisting of four blood samples and four saliva samples) applied in 20 patients (Table 1). Studies with a baseline PC or a peak of PC in low-dose ACTH stimulation test higher than 18 µg/dL were considered normal (without AI). According to this classification, 8 studies were normal and AI was identified in 22.
| Table 1: Subject characteristic and cortisol measures (µg/dL) in ACTH test. NR: no result available. | ||||||||||
| ID study | Gender | Age | PC 0 | PC 30 | PC 60 | PC 90 | SC 0 | SC 30 | SC 60 | SC 90 |
| 1 | M | 8y 5m | 14,72 | 12,62 | 8,08 | 6,06 | 0,413 | 0,404 | 0,302 | 0,260 |
| 2 | M | 14y 6m | 14,18 | 9,94 | 6,49 | 5 | 0,44 | 0,30 | 0,20 | 0,170 |
| 3 | M | 15y 6m | 19,42 | 14,72 | 11,84 | 12,64 | 0,996 | 0,627 | 0,482 | 0,456 |
| 4 | F | 11y 7m | 8,19 | 4,72 | 3,34 | 3 | 0,141 | 0,164 | 0,294 | 0,110 |
| 5 | F | 12y | 9,8 | 18,59 | 9,14 | 5,04 | 0,263 | 0,617 | 0,524 | 0,511 |
| 6 | F | 10y 4m | 9,08 | 6,03 | 6,27 | 5,32 | 0,174 | 0,149 | 0,123 | 0,110 |
| 7 | F | 10y 7m | 15,29 | 14,11 | 21,36 | 15,99 | 0,766 | 0,439 | 0,689 | 0,436 |
| 8 | F | 14y 11m | 11,04 | 6,2 | 4,15 | 2,94 | 0,326 | 0,335 | 0,257 | 0,185 |
| 9 | F | 16y 2m | 11,54 | 9,9 | 7,53 | 6,35 | 0,42 | 0,439 | 0,285 | 0,289 |
| 10 | F | 10y 2m | 0,49 | 0,73 | 0,47 | 0,42 | 0,55 | 0,44 | 0,34 | 0,270 |
| 11 | F | 10y 6m | 12,4 | NR | 14,06 | NR | 0,34 | 0,283 | 0,238 | NR |
| 12 | F | 10y 11m | 18,58 | 10,21 | 7,01 | 7,08 | 0,606 | 0,414 | 0,246 | 0,258 |
| 13 | F | 14y 8m | 19,6 | 16,32 | 11,16 | 9,23 | 0,891 | 0,682 | 0,427 | 0,338 |
| 14 | F | 12y 6m | 2,34 | 3,65 | 2,78 | 2,53 | NR | 0,35 | 0,25 | 0,21 |
| 15 | F | 12y 9m | 17,1 | 15,5 | 12,81 | 10,93 | 0,425 | NR | 0,331 | 0,285 |
| 16 | F | 13y | 6,12 | 6,28 | 4,54 | 4,02 | 0,227 | 0,196 | 0,136 | 0,209 |
| 17 | F | 13y 5m | 14,44 | 14,53 | 11,16 | 8,23 | 0,344 | 0,372 | 0,203 | 0,127 |
| 18 | F | 3y 8m | 14,36 | 13,42 | 10,57 | 7,49 | 0,28 | 0,256 | 0,207 | 0,168 |
| 19 | F | 3y 11m | 15,2 | 13,94 | 9,28 | 9,51 | 0,354 | 0,331 | 0,258 | 0,233 |
| 20 | F | 4y 5m | 19,15 | 17,15 | 11,8 | 9,05 | 0,291 | 0,273 | 0,107 | 0,057 |
| 21 | F | 9y 5m | 0,05 | 0,08 | 0,05 | 0,05 | Insufficient sample | < 0,018 | < 0,018 | < 0,018 |
| 22 | M | 6y | 24,3 | 18,7 | 10,2 | 9,9 | 0,74 | NR | NR | NR |
| 23 | M | 14y 5m | 13,6 | 15,32 | 10,58 | 7,64 | 0,355 | 0,471 | 0,217 | 0,112 |
| 24 | M | 14y 9m | 11 | 9,22 | 5,81 | 4,48 | 0,181 | 0,164 | 0,08 | 0,111 |
| 25 | F | 12y 8m | 5,96 | 3,39 | 2,22 | 2,5 | 0,092 | 0,054 | 0,054 | 0,054 |
| 26 | F | 13y 2m | 10,3 | 8,07 | 6 | 18,4 | 0,101 | 0,069 | 0,082 | 0,22 |
| 27 | F | 7y 6m | 4,76 | 11,12 | 5,51 | 3,63 | 0,07 | 0,225 | 0,225 | 0,217 |
| 28 | F | 9y 5m | 1,44 | 2,03 | 1,18 | 0,91 | 0,614 | 0,333 | NR | 0,175 |
| 29 | F | 5y 2m | 15,03 | 18,67 | 13,61 | 11,34 | 0,782 | 1,04 | 0,64 | 0,489 |
| 30 | F | 16y 2m | 0,81 | 1,2 | 0,72 | 0,51 | 0,061 | 0,041 | 0,049 | 0,036 |
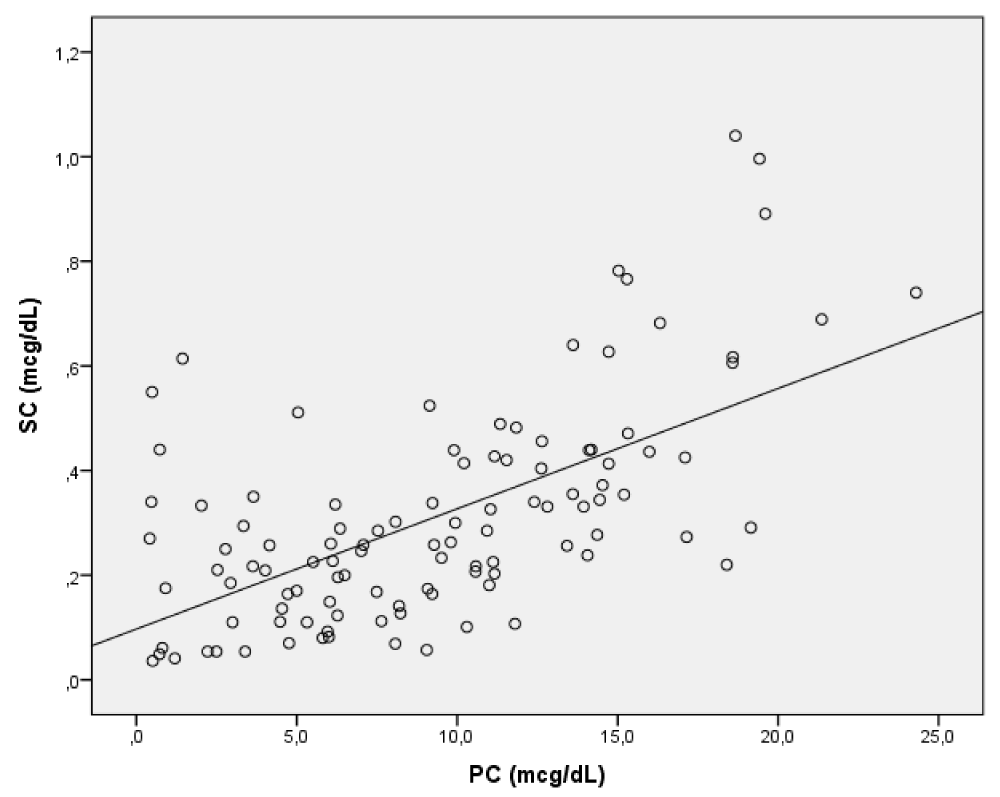
Pearson’s linear regression analysis showed a positive correlation between SC and PC (Figure 1) (r = 0.618, p < 0.001).
Figure 1: Correlation between plasma (PC) and salivary cortisol (SC).
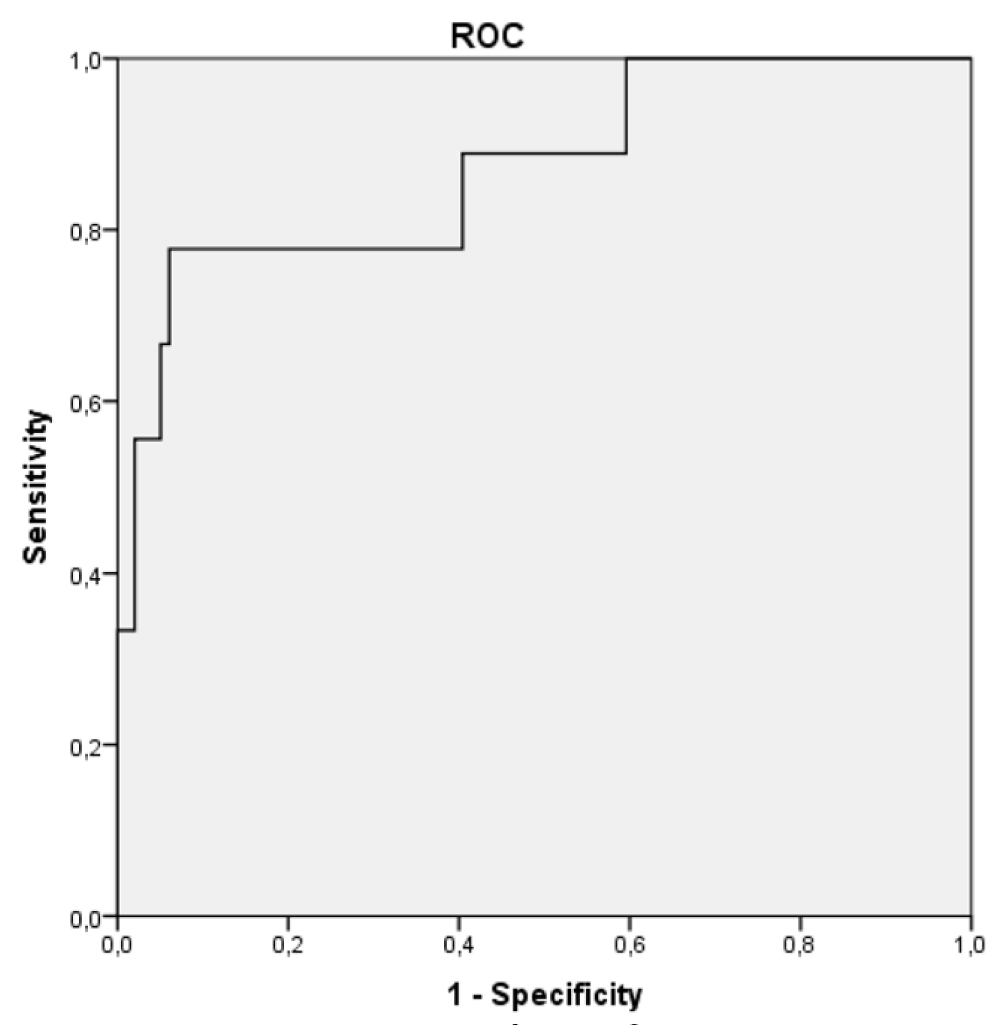
The cut-off for peak salivary cortisol (during ACTH stimulation test) that allowed us to differentiate the two populations (healthy children versus patients with AI) with the ROC analysis, taking into account the maximum values of SC of each study was 0.61 µg/dL (Figure 2) with a sensitivity and specificity of 93.94% and 66.67%, respectively.
Figure 2: ROC curve for salivary cortisol to define adrenal sufficiency.
Adrenal insufficiency is a life-threatening condition requiring prompt diagnosis, and currently, the main cause in the paediatric population is iatrogenic, result of prolonged treatments with high-dose exogenous corticosteroids. Laboratory evaluation of patients with suspected AI begins with the measurement of total baseline PC, but this is not sufficient to accurately diagnose this condition. For this reason, the most widely used adrenal function indicator in clinical practice is the determination of total PC during ACTH stimulation. Yet PC is not the best tool because 80% - 90% of circulating cortisol is bound to CBG and measurement of plasma cortisol is compromised by conditions altering CBG levels [8,9]. Although the exact mechanism of cortisol secretion in saliva is unclear, salivary cortisol reflects circulating free cortisol levels better than total plasma cortisol (SC is not influenced by CBG levels, only free plasma cortisol passes into saliva). In cirrhotic patients, free cortisol seems to be more strongly correlated with salivary cortisol than with total plasma cortisol (Spearman coefficient 0.91 vs. 0.76, respectively, p < 0.001) [10]. Consequently, the measurement of salivary cortisol levels can be used as an alternative technique in the diagnosis of adrenal disease. Saliva samples can easily be obtained in a non-invasive way while avoiding stress-related cortisol release that may occur during blood sampling. Furthermore, it is an accurate tool to test adrenocortical function under basal and stimulated conditions, especially in infants and young children, and offers a viable alternative to test plasma cortisol levels.
We included children over 2 years old, because under this age the amount of saliva produced seems to be more variable and unpredictable [11]. We chose a saliva testing device because it is considered the best method to obtain salivary cortisol [12,13]. Intramuscular ACTH administration was used to evaluate the correlation between PC and SC in order to avoid intravascular administration in the future, if SC was optimal.
Some studies have shown that plasma cortisol passes and concentrates in the saliva in approximately 1 to 5 minutes (reaching a plateau in 6-12 minutes), after IV injection of cortisol or after ACTH stimulation test [8,14]. All these adult’s studies showed a correlation between PC and SC levels (r = 0.83-0.932), but a good correlation during low-dose ACTH test between these two parameters was only found in one paediatric study [8]. In critically ill children admitted in the Paediatric Intensive Care Unit, there was a significant positive correlation between total plasma cortisol and salivary cortisol concentrations at baseline (r = 0.67; p < 0.0001) and following the ACTH test (r = 0.41; p < 0.02) [1]. However, in athletes, SC did not correlate with PC, but this difference may be due to the different methods used and time of collection [15]. In children with hyperactivity attention deficit disorder, SC was higher than in controls, which is a good stress indicator [16]. SC was not useful in preterm infants probably due to small sample size [17].
Our study showed a highly positive correlation between salivary and total plasma cortisol levels in children with adrenal insufficiency caused by long-term steroid use, which is in agreement with previously published studies [1,8]. Furthermore, this positive correlation has been shown in adults with Cushing’s syndrome and end-stage renal disease [1,18,19]. These findings reveal that the pharmacodynamics of cortisol in the paediatric population may not be different than in adults, and SC becomes a tool that could be applied to a range of disease settings to monitor adrenocortical function, and is also feasible in critically ill patients, especially in children.
Several published studies have attempted to identify cut-off levels for SC in the diagnosis of Cushing syndrome, in both adults and the paediatric population [8]. In Cushing patients, there is a good concordance with free urine cortisol and SC can be offered as an alternative better screening study [20,21]. However, data regarding the value of SC in the diagnosis of AI in children are scarce [2,22,23]. In the current study, a SC peak during low-dose ACTH test higher than 0.61 µg/dL identified patient without AI, in agreement with other published studies.
This study has several limitations: the sample size can be considered limited and the samples were collected during different seasons. Additional studies including patients with adrenal insufficiency are needed to confirm these findings (especially to assess the diagnostic value of the suggested cut-off levels) in a larger cohort and to determine the validity of measuring salivary cortisol in other adrenal diseases.
Salivary cortisol is a non-invasive, easily applicable test.
Cortisol levels in saliva and plasma are highly correlated and a cut-off for peak SC during low-dose ACTH stimulation test of 0.61 µg/dL is useful to rule out adrenal insufficiency. It could replace plasma cortisol as a screening tool for the diagnosis of secondary AI.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This project was supported by a grant from Foundation Parc Taulí (CEIC 2014510).
- Balbão VM, Costa MM, Castro M, Carlotti AP. Evaluation of adrenal function in critically ill children. Clin Endocrinol (Oxf). 2014; 81: 559-565. PubMed: https://pubmed.ncbi.nlm.nih.gov/24588209/
- Elbuken G, Tanriverdi F, Karaca Z, Kula M, Gokahmetoglu S, et al. Comparison of salivary and calculated free cortisol levels during low and standard dose of ACTH stimulation tests in healthy volunteers. Endocrine. 2015; 48: 439-443. PubMed: https://pubmed.ncbi.nlm.nih.gov/25115637/
- Chiappin S, Antonelli G, Gatti R, De Palo EF. Saliva specimen: a new laboratory tool for diagnostic and basic investigation. Clin Chim Acta. 2007; 383: 30-40. PubMed: https://pubmed.ncbi.nlm.nih.gov/17512510/
- Lewis JG. Steroid analysis in saliva: an overview. Clin Biochem Rev. 2006; 27: 139-146. PubMed: https://pubmed.ncbi.nlm.nih.gov/17268582/
- Arafah BM, Nishiyama FJ, Tlaygeh H, Hejal R. Measurement of salivary cortisol concentration in the assessment of adrenal function in critically ill subjects: a surrogate marker of the circulating free cortisol. J Clin Endocrinol Metab. 2007; 92: 2965-2971. PubMed: https://pubmed.ncbi.nlm.nih.gov/17535998/
- Törnhage CJ. Salivary cortisol for assessment of hypothalamic-pituitary-adrenal axis function. Neuroimmunomodulation. 2009; 16: 284-289. PubMed: https://pubmed.ncbi.nlm.nih.gov/19571589/
- Jessop DS, Turner-Cobb JM. Measurement and meaning of salivary cortisol: a focus on health and disease in children. Stress. 2008; 11: 1-14. PubMed: https://pubmed.ncbi.nlm.nih.gov/17853059/
- Cetinkaya S, Ozon A, Yordam N. Diagnostic value of salivary cortisol in children with abnormal adrenal cortex functions. Horm Res. 2007; 67: 301-306. PubMed: https://pubmed.ncbi.nlm.nih.gov/17337901/
- Marcus-Perlman Y, Tordjman K, Greenman Y, Limor R, Shenkerman G, Osher E, et al. Low-dose ACTH (1 microg) salivary test: a potential alternative to the classical blood test. Clin Endocrinol (Oxf). 2006;64: 215-218. PubMed: https://pubmed.ncbi.nlm.nih.gov/16430723/
- Galbois A, Rudler M, Massard J, Fulla Y, Bennani A, et al. Assessment of adrenal function in cirrhotic patients: salivary cortisol should be preferred. J Hepatol. 2010; 52: 839-845. PubMed: https://pubmed.ncbi.nlm.nih.gov/20385427/
- Maguire AM, Cowell CT. Salivary cortisol to assess the hypothalamic-pituitary-adrenal axis in healthy children under 3 years old. J Pediatr (Rio J). 2007; 83: 383-384. PubMed: https://pubmed.ncbi.nlm.nih.gov/17676242/
- Tryphonopoulos PD, Letourneau N, Azar R. Approaches to salivary cortisol collection and analysis in infants. Biol Res Nurs. 2014; 16: 398-408. PubMed: https://pubmed.ncbi.nlm.nih.gov/24136995/
- Hanrahan K, McCarthy AM, Kleiber C, Lutgendorf S, Tsalikian E. Strategies for salivary cortisol collection and analysis in research with children. Appl Nurs Res. 2006; 19: 95-101. PubMed: https://pubmed.ncbi.nlm.nih.gov/16728293/
- Vining RF, McGinley RA. The measurement of hormones in saliva: possibilities and pitfalls. J Steroid Biochem. 1987; 27: 81-94. PubMed: https://pubmed.ncbi.nlm.nih.gov/3320544/
- Cevada T, Vasques PE, Moraes H, Deslandes A. Salivary cortisol levels in athletes and nonathletes: a systematic review. Horm Metab Res. 2014; 46: 905-910. PubMed: https://pubmed.ncbi.nlm.nih.gov/25230328/
- Aguilar Cordero MJ, Sánchez López AM, Mur Villar N, García García I, Rodríguez López MA, et al. [Salivary cortisol as an indicator of physological stress in children and adults; a systematic review]. Nutr Hosp. 2014; 29: 960-968. PubMed: https://pubmed.ncbi.nlm.nih.gov/24951973/
- Maas C, Ringwald C, Weber K, Engel C, Poets CF, et al. Relationship of salivary and plasma cortisol levels in preterm infants: results of a prospective observational study and systematic review of the literature. Neonatology. 2014; 105: 312-318. PubMed: https://pubmed.ncbi.nlm.nih.gov/24603497/
- Raff H, Raff JL, Findling JW. Late-night salivary cortisol as a screening test for Cushing's syndrome. J Clin Endocrinol Metab. 1998; 83: 2681-2686. PubMed: https://pubmed.ncbi.nlm.nih.gov/9709931/
- Arregger AL, Cardoso EM, Tumilasci O, Contreras LN. Diagnostic value of salivary cortisol in end stage renal disease. Steroids. 2008; 73: 77-82. PubMed: https://pubmed.ncbi.nlm.nih.gov/17945323/
- Doi SA, Clark J, Russell AW. Concordance of the late night salivary cortisol in patients with Cushing's syndrome and elevated urine-free cortisol. Endocrine. 2013; 43: 327-333. PubMed: https://pubmed.ncbi.nlm.nih.gov/23238876/
- Elamin MB, Murad MH, Mullan R, Erickson D, Harris K, et al. Accuracy of diagnostic tests for Cushing's syndrome: a systematic review and metaanalyses. J Clin Endocrinol Metab. 2008; 93: 1553-1562. PubMed: https://pubmed.ncbi.nlm.nih.gov/18334594/
- le Roux CW, Chapman GA, Kong WM, Dhillo WS, Jones J, et al. Free cortisol index is better than serum total cortisol in determining hypothalamic-pituitary-adrenal status in patients undergoing surgery. J Clin Endocrinol Metab. 2003; 88: 2045-2048. PubMed: https://pubmed.ncbi.nlm.nih.gov/12727952/
- Poomthavorn P, Lertbunrian R, Preutthipan A, Sriphrapradang A, Khlairit P, et al. Serum free cortisol index, free cortisol, and total cortisol in critically ill children. Intensive Care Med. 2009; 35: 1281-1285. PubMed: https://pubmed.ncbi.nlm.nih.gov/19352620/